Abstract
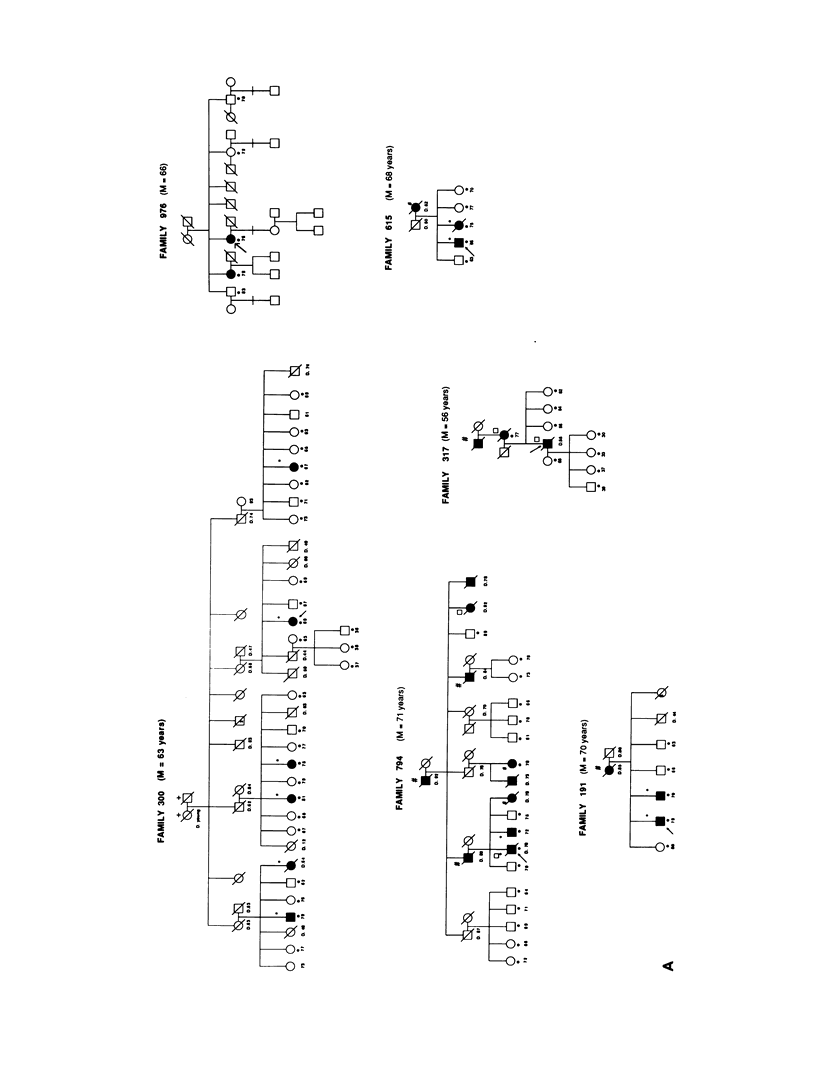
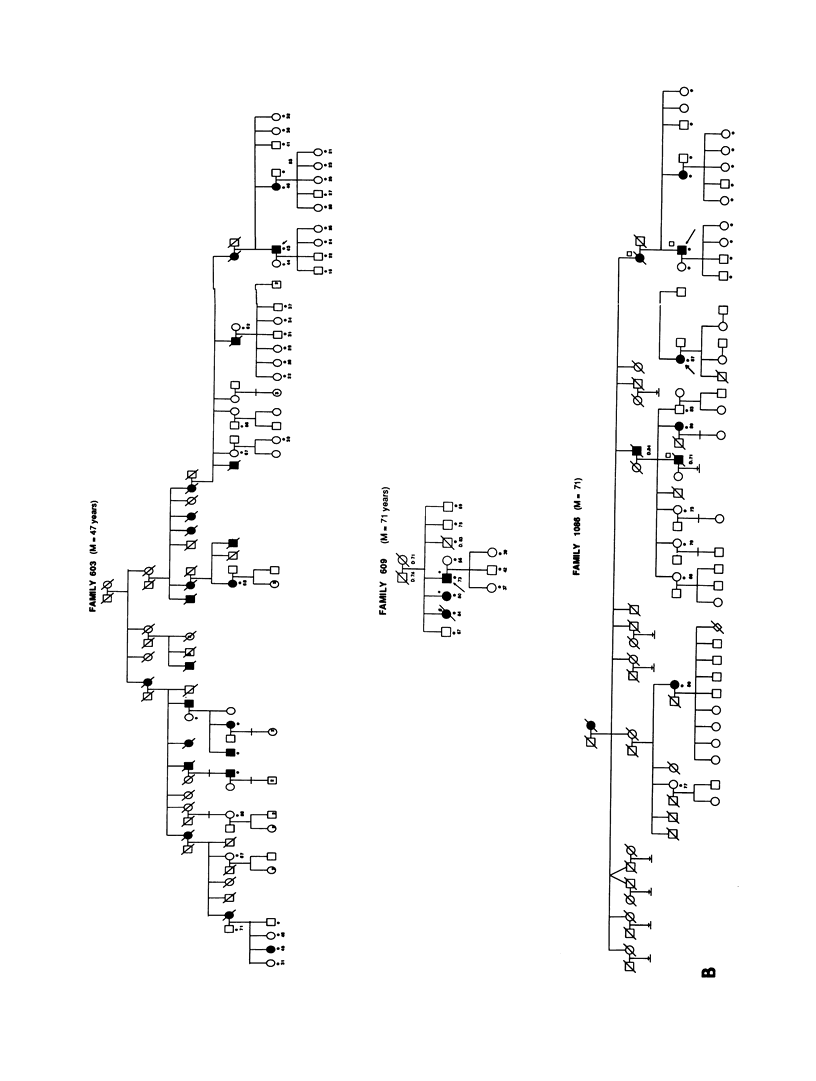
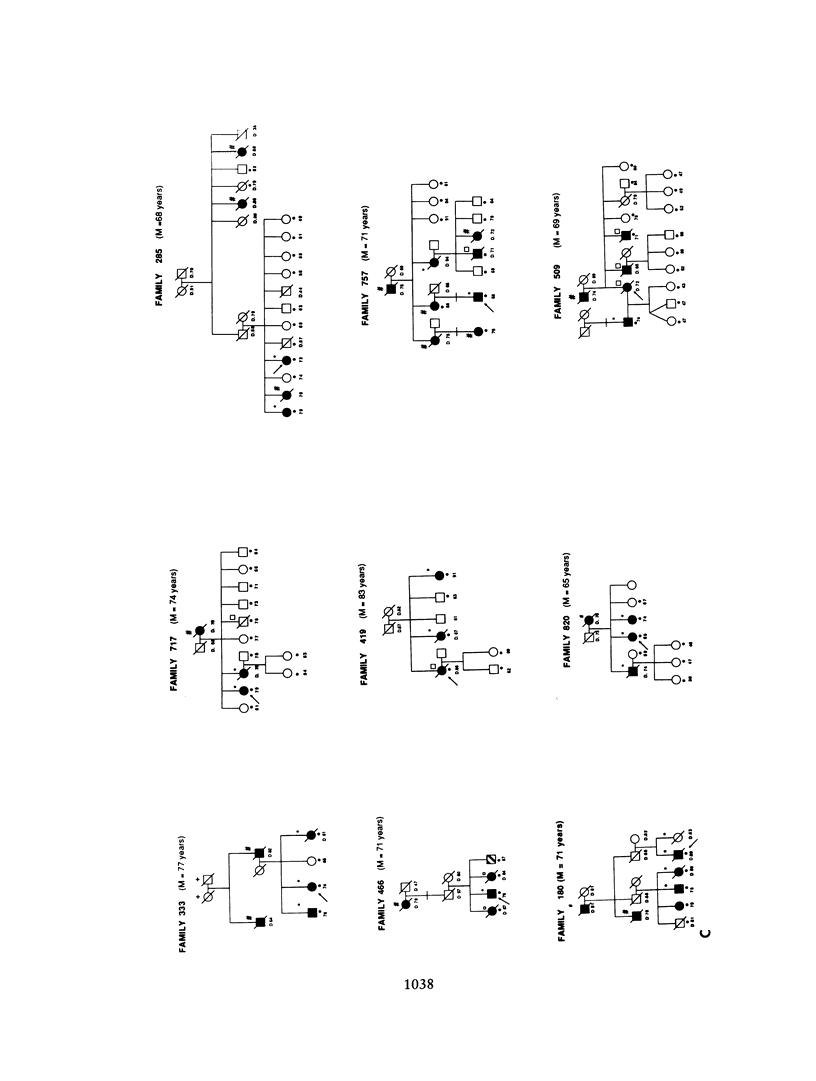
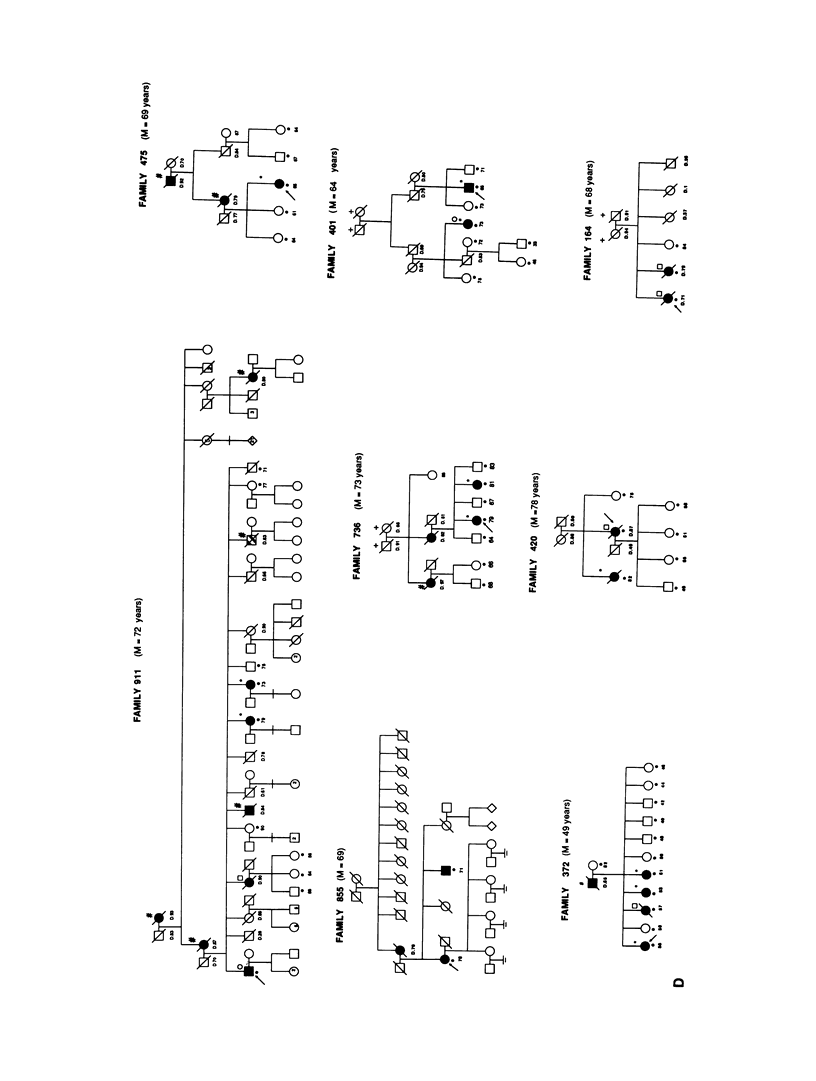
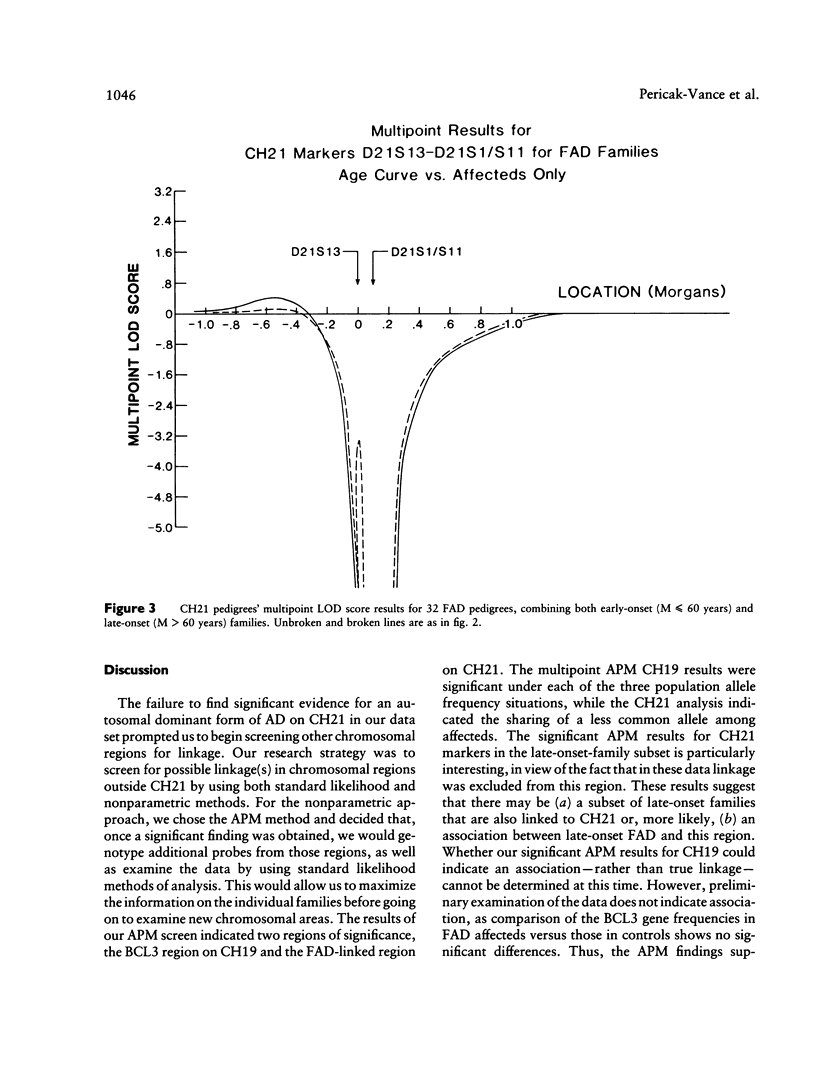
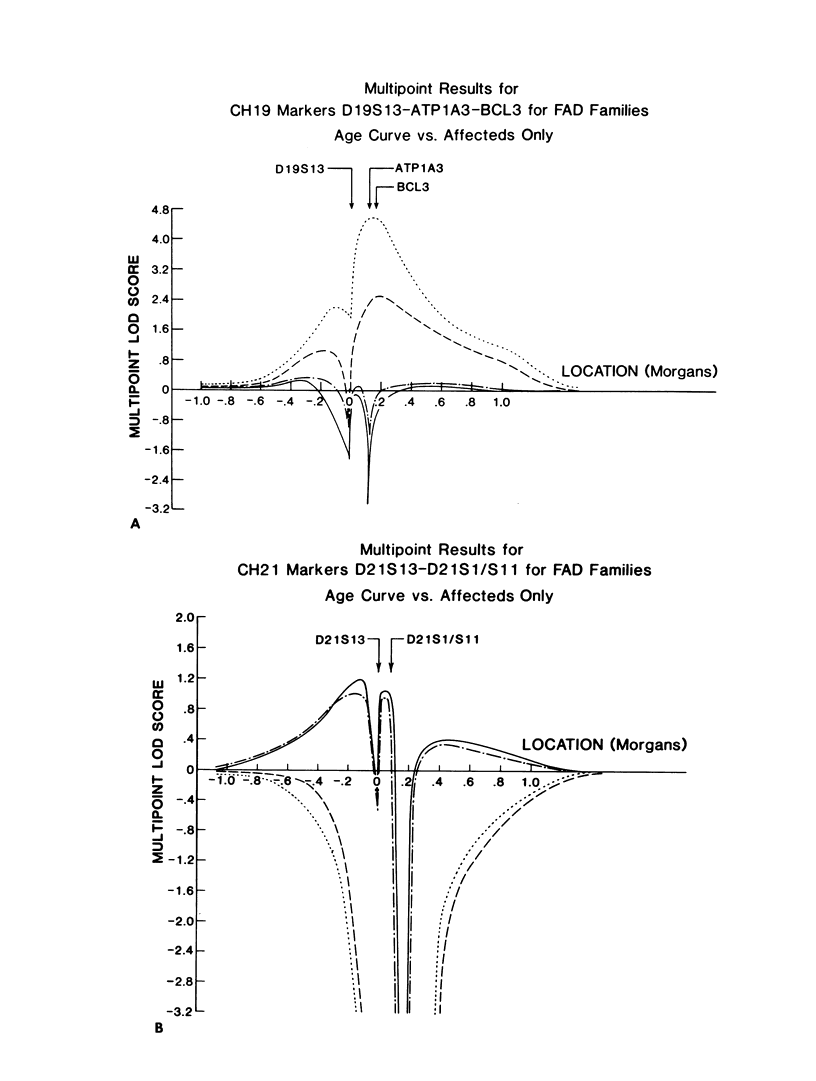
A genetic component in the etiology of Alzheimer disease (AD) has been supported by indirect evidence for several years, with autosomal dominant inheritance with age-dependent penetrance being suggested to explain the familial aggregation of affecteds. St. George Hyslop et al. reported linkage of familial AD (FAD) in four early-onset families (mean age at onset [M] < 50 years). Subsequent studies have been inconsistent in their results; Goate et al. also reported positive lod scores. However, both Pericak-Vance et al.'s study of a series of mainly late-onset FAD families (M > 60 years) and Schellenberg et al.'s study failed to confirm linkage to chromosome 21 (CH21). These various studies suggest the possibility of genetic heterogeneity, with some families linked to CH21 and others unlocalized. Recently, St. George Hyslop et al. extended their analysis to include additional families. The extended analyses supported their earlier finding of linkage to CH21, while showing strong evidence of heterogeneity between early-onset (M < 65 years) and late-onset (M > 60 years) FAD families. Because our families did not show linkage to CH21, we undertook a genomic search for an additional locus for FAD. Because of both the confounding factor of late age at onset of FAD and the lack of clear evidence of Mendelian transmission in some of our families, we employed the affected-pedigree-member (APM) method of linkage analysis as an initial screen for possible linkage. Using this method, we identified two regions suggesting linkage: the proximal long arm of chromosome 19 (CH19) and the CH21 region of FAD linkage reported by St. George Hyslop et al. Application of standard likelihood (LOD score) analysis to these data support the possibility of an FAD gene located on CH19, particularly in the late-onset FAD families. These data further suggest genetic heterogeneity and delineate this region of CH19 as an area needing additional investigation in FAD.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett R. J., Pericak-Vance M. A., Yamaoka L., Gilbert J., Herbstreith M., Hung W. Y., Lee J. E., Mohandas T., Bruns G., Laberge C. A new probe for the diagnosis of myotonic muscular dystrophy. Science. 1987 Mar 27;235(4796):1648–1650. doi: 10.1126/science.3029876. [DOI] [PubMed] [Google Scholar]
- Breitner J. C., Silverman J. M., Mohs R. C., Davis K. L. Familial aggregation in Alzheimer's disease: comparison of risk among relatives of early-and late-onset cases, and among male and female relatives in successive generations. Neurology. 1988 Feb;38(2):207–212. doi: 10.1212/wnl.38.2.207. [DOI] [PubMed] [Google Scholar]
- Goate A. M., Haynes A. R., Owen M. J., Farrall M., James L. A., Lai L. Y., Mullan M. J., Roques P., Rossor M. N., Williamson R. Predisposing locus for Alzheimer's disease on chromosome 21. Lancet. 1989 Feb 18;1(8634):352–355. doi: 10.1016/s0140-6736(89)91725-x. [DOI] [PubMed] [Google Scholar]
- Haynes C., Pericak-Vance M., Dawson D. Analysis of Huntington disease linkage and age-of-onset distributions. Genet Epidemiol Suppl. 1986;1:235–239. doi: 10.1002/gepi.1370030736. [DOI] [PubMed] [Google Scholar]
- Heyman A., Wilkinson W. E., Stafford J. A., Helms M. J., Sigmon A. H., Weinberg T. Alzheimer's disease: a study of epidemiological aspects. Ann Neurol. 1984 Apr;15(4):335–341. doi: 10.1002/ana.410150406. [DOI] [PubMed] [Google Scholar]
- Keats B., Ott J., Conneally M. Report of the committee on linkage and gene order. Cytogenet Cell Genet. 1989;51(1-4):459–502. doi: 10.1159/000132805. [DOI] [PubMed] [Google Scholar]
- Kidd K. K., Bowcock A. M., Schmidtke J., Track R. K., Ricciuti F., Hutchings G., Bale A., Pearson P., Willard H. F., Gelernter J. Report of the DNA committee and catalogs of cloned and mapped genes and DNA polymorphisms. Cytogenet Cell Genet. 1989;51(1-4):622–947. doi: 10.1159/000132810. [DOI] [PubMed] [Google Scholar]
- Lange K., Weeks D. E. Linkage methods for identifying genetic risk factors. World Rev Nutr Diet. 1990;63:236–249. doi: 10.1159/000418512. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M. Easy calculations of lod scores and genetic risks on small computers. Am J Hum Genet. 1984 Mar;36(2):460–465. [PMC free article] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON N. E. The detection and estimation of linkage between the genes for elliptocytosis and the Rh blood type. Am J Hum Genet. 1956 Jun;8(2):80–96. [PMC free article] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E. M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mohs R. C., Breitner J. C., Silverman J. M., Davis K. L. Alzheimer's disease. Morbid risk among first-degree relatives approximates 50% by 90 years of age. Arch Gen Psychiatry. 1987 May;44(5):405–408. doi: 10.1001/archpsyc.1987.01800170019003. [DOI] [PubMed] [Google Scholar]
- Pericak-Vance M. A., Hung W. Y., Yamaoka L., Haynes C., Bartlett R. J., Vance J. M., Lee J., Siddique T., Gaskell P. C., Stajich J. Systematic gene mapping in man: data management considerations. Aust Paediatr J. 1988;24 (Suppl 1):87–89. [PubMed] [Google Scholar]
- Pericak-Vance M. A., Meyers D. A. Genetic analysis workshop IV: Huntington disease linkage analysis summary. Genet Epidemiol Suppl. 1986;1:197–209. doi: 10.1002/gepi.1370030731. [DOI] [PubMed] [Google Scholar]
- Pericak-Vance M. A., Yamaoka L. H., Assinder R. I., Hung W. Y., Bartlett R. J., Stajich J. M., Gaskell P. C., Ross D. A., Sherman S., Fey G. H. Tight linkage of apolipoprotein C2 to myotonic dystrophy on chromosome 19. Neurology. 1986 Nov;36(11):1418–1423. doi: 10.1212/wnl.36.11.1418. [DOI] [PubMed] [Google Scholar]
- Pericak-Vance M. A., Yamaoka L. H., Haynes C. S., Speer M. C., Haines J. L., Gaskell P. C., Hung W. Y., Clark C. M., Heyman A. L., Trofatter J. A. Genetic linkage studies in Alzheimer's disease families. Exp Neurol. 1988 Dec;102(3):271–279. doi: 10.1016/0014-4886(88)90220-8. [DOI] [PubMed] [Google Scholar]
- Schellenberg G. D., Bird T. D., Wijsman E. M., Moore D. K., Boehnke M., Bryant E. M., Lampe T. H., Nochlin D., Sumi S. M., Deeb S. S. Absence of linkage of chromosome 21q21 markers to familial Alzheimer's disease. Science. 1988 Sep 16;241(4872):1507–1510. doi: 10.1126/science.3420406. [DOI] [PubMed] [Google Scholar]
- Schellenberg G. D., Deeb S. S., Boehnke M., Bryant E. M., Martin G. M., Lampe T. H., Bird T. D. Association of an apolipoprotein CII allele with familial dementia of the Alzheimer type. J Neurogenet. 1987 Apr;4(2-3):97–108. [PubMed] [Google Scholar]
- St George-Hyslop P. H., Haines J. L., Farrer L. A., Polinsky R., Van Broeckhoven C., Goate A., McLachlan D. R., Orr H., Bruni A. C., Sorbi S. Genetic linkage studies suggest that Alzheimer's disease is not a single homogeneous disorder. Nature. 1990 Sep 13;347(6289):194–197. doi: 10.1038/347194a0. [DOI] [PubMed] [Google Scholar]
- St George-Hyslop P. H., Tanzi R. E., Polinsky R. J., Haines J. L., Nee L., Watkins P. C., Myers R. H., Feldman R. G., Pollen D., Drachman D. The genetic defect causing familial Alzheimer's disease maps on chromosome 21. Science. 1987 Feb 20;235(4791):885–890. doi: 10.1126/science.2880399. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Haines J. L., Watkins P. C., Stewart G. D., Wallace M. R., Hallewell R., Wong C., Wexler N. S., Conneally P. M., Gusella J. F. Genetic linkage map of human chromosome 21. Genomics. 1988 Aug;3(2):129–136. doi: 10.1016/0888-7543(88)90143-7. [DOI] [PubMed] [Google Scholar]
- Vance J. M., Barker D., Yamaoka L. H., Stajich J. M., Loprest L., Hung W. Y., Fischbeck K., Roses A. D., Pericak-Vance M. A. Localization of Charcot-Marie-Tooth disease type 1a (CMT1A) to chromosome 17p11.2. Genomics. 1991 Apr;9(4):623–628. doi: 10.1016/0888-7543(91)90355-i. [DOI] [PubMed] [Google Scholar]
- Weeks D. E., Lange K. The affected-pedigree-member method of linkage analysis. Am J Hum Genet. 1988 Feb;42(2):315–326. [PMC free article] [PubMed] [Google Scholar]
- Yamaoka L. H., Pericak-Vance M. A., Speer M. C., Gaskell P. C., Jr, Stajich J., Haynes C., Hung W. Y., Laberge C., Thibault M. C., Mathieu J. Tight linkage of creatine kinase (CKMM) to myotonic dystrophy on chromosome 19. Neurology. 1990 Feb;40(2):222–226. doi: 10.1212/wnl.40.2.222. [DOI] [PubMed] [Google Scholar]


