Abstract
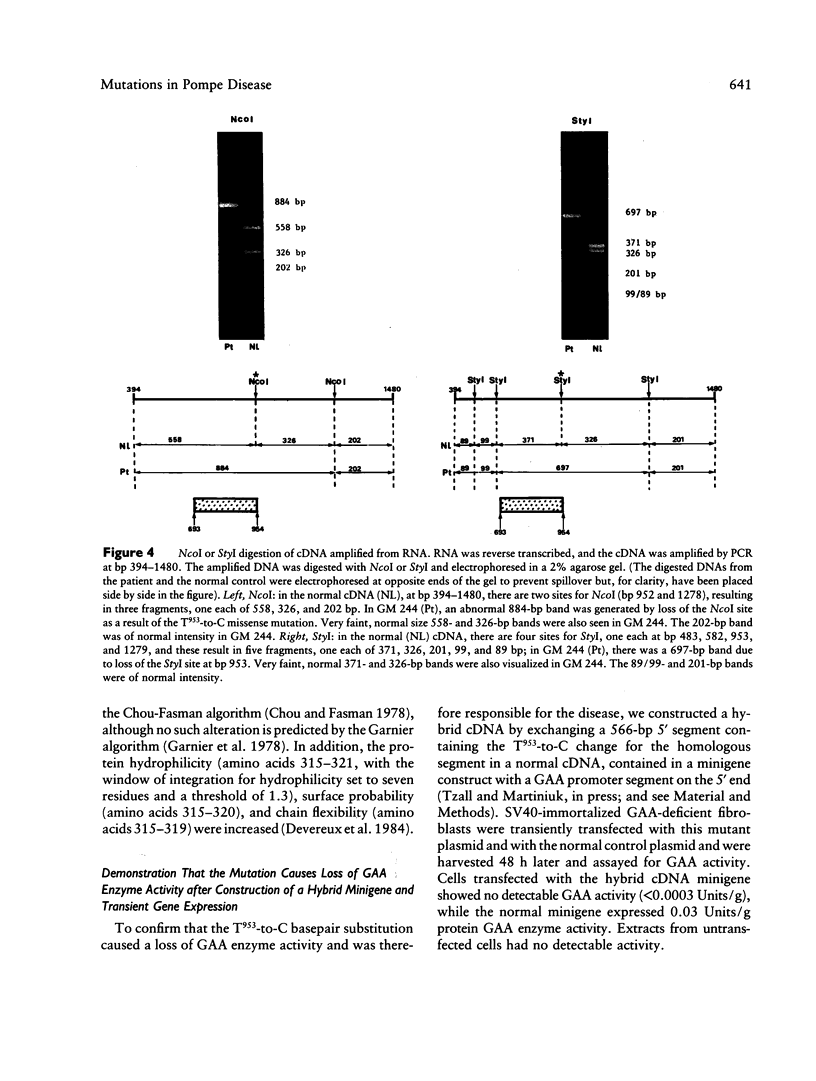
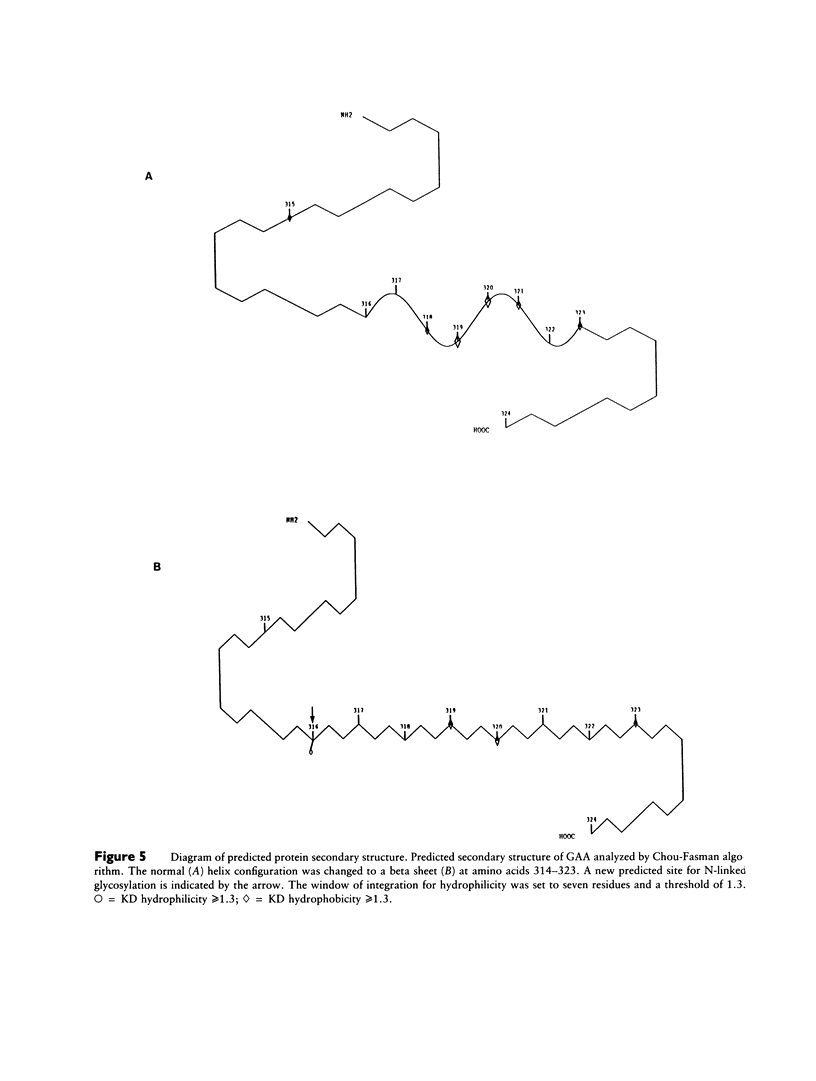
Infantile-onset glycogen storage disease type II, or Pompe disease, results from a genetic deficiency of the lysosomal enzyme acid alpha glucosidase (GAA). Sequencing of the cDNA from a cell line (GM 244) derived from a patient with Pompe disease demonstrated a T953-to-C transition that predicted a methionine-to-threonine substitution at codon 318. The basepair substitution resulted in loss of restriction-endonuclease sites for NcoI and StyI. Analysis of genomic DNA revealed both a normal and an abnormal NcoI fragment, indicating that the patient was a genetic compound. NcoI and StyI digestion of cDNA, amplified by PCR from reverse-transcribed RNA, demonstrated that greater than 95% of the GAA mRNA in GM 244 was derived from the allele carrying the missense mutation. The missense mutation was uncommon, since it was not detected in 37 additional GAA-deficient chromosomes, as determined by digestion of genomic DNA with NcoI and hybridization. The amino acid substitution predicts a new potential site for N-linked glycosylation, as well as major changes in secondary structure of the protein. We could confirm that the mutation was responsible for the enzyme deficiency by demonstrating that a hybrid minigene containing the mutation did not express GAA enzyme activity after transient gene expression. We have therefore now provided the first identification of a single-basepair missense mutation in a patient with Pompe disease and furthermore have demonstrated that the patient is a genetic compound with the second allele barely expressing mRNA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beratis N. G., LaBadie G. U., Hirschhorn K. Characterization of the molecular defect in infantile and adult acid alpha-glucosidase deficiency fibroblasts. J Clin Invest. 1978 Dec;62(6):1264–1274. doi: 10.1172/JCI109247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. I., Brown D. H., Jeffrey P. L. Simultaneous absence of alpha-1,4-glucosidase and alpha-1,6-glucosidase activities (pH 4) in tissues of children with type II glycogen storage disease. Biochemistry. 1970 Mar 17;9(6):1423–1428. doi: 10.1021/bi00808a017. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cooper D. N., Krawczak M. The mutational spectrum of single base-pair substitutions causing human genetic disease: patterns and predictions. Hum Genet. 1990 Jun;85(1):55–74. doi: 10.1007/BF00276326. [DOI] [PubMed] [Google Scholar]
- Courtecuissf V., Royer P., Habib R., Monnier C., Demos J. Glycogenose musculaire par deficit d'alpha-1-4-glucosidase simulant une dystrophie musculaire progressive. (Etude clinique et enzymatique. Microscopie optique electronique) Arch Fr Pediatr. 1965 Dec;22(10):1153–1164. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A. G., Gomez M. R., Seybold M. E., Lambert E. H. The spectrum and diagnosis of acid maltase deficiency. Neurology. 1973 Jan;23(1):95–106. doi: 10.1212/wnl.23.1.95. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- HERS H. G. alpha-Glucosidase deficiency in generalized glycogenstorage disease (Pompe's disease). Biochem J. 1963 Jan;86:11–16. doi: 10.1042/bj0860011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Hidaka Y., Tarlé S. A., Fujimori S., Kamatani N., Kelley W. N., Palella T. D. Human adenine phosphoribosyltransferase deficiency. Demonstration of a single mutant allele common to the Japanese. J Clin Invest. 1988 Mar;81(3):945–950. doi: 10.1172/JCI113408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilkens J., Tager J. M., Buijs F., Brouwer-Kelder B., Van Thienen G. M., Tegelaers F. P., Hilgers J. Monoclonal antibodies against human acid alpha-glucosidase. Biochim Biophys Acta. 1981 Nov 18;678(1):7–11. doi: 10.1016/0304-4165(81)90041-6. [DOI] [PubMed] [Google Scholar]
- Hoefsloot L. H., Hoogeveen-Westerveld M., Kroos M. A., van Beeumen J., Reuser A. J., Oostra B. A. Primary structure and processing of lysosomal alpha-glucosidase; homology with the intestinal sucrase-isomaltase complex. EMBO J. 1988 Jun;7(6):1697–1704. doi: 10.1002/j.1460-2075.1988.tb02998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefsloot L. H., van der Ploeg A. T., Kroos M. A., Hoogeveen-Westerveld M., Oostra B. A., Reuser A. J. Adult and infantile glycogenosis type II in one family, explained by allelic diversity. Am J Hum Genet. 1990 Jan;46(1):45–52. [PMC free article] [PubMed] [Google Scholar]
- Martiniuk F., Honig J., Hirschhorn R. Further studies of the structure of human placental acid alpha-glucosidase. Arch Biochem Biophys. 1984 Jun;231(2):454–460. doi: 10.1016/0003-9861(84)90408-9. [DOI] [PubMed] [Google Scholar]
- Martiniuk F., Mehler M., Pellicer A., Tzall S., La Badie G., Hobart C., Ellenbogen A., Hirschhorn R. Isolation of a cDNA for human acid alpha-glucosidase and detection of genetic heterogeneity for mRNA in three alpha-glucosidase-deficient patients. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9641–9644. doi: 10.1073/pnas.83.24.9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiniuk F., Mehler M., Tzall S., Meredith G., Hirschhorn R. Extensive genetic heterogeneity in patients with acid alpha glucosidase deficiency as detected by abnormalities of DNA and mRNA. Am J Hum Genet. 1990 Jul;47(1):73–78. [PMC free article] [PubMed] [Google Scholar]
- Mehler M., DiMauro S. Residual acid maltase activity in late-onset acid maltase deficiency. Neurology. 1977 Feb;27(2):178–184. doi: 10.1212/wnl.27.2.178. [DOI] [PubMed] [Google Scholar]
- Oude Elferink R. P., Van Doorn-Van Wakeren J., Strijland A., Reuser A. J., Tager J. M. Biosynthesis and intracellular transport of alpha-glucosidase and cathepsin D in normal and mutant human fibroblasts. Eur J Biochem. 1985 Nov 15;153(1):55–63. doi: 10.1111/j.1432-1033.1985.tb09266.x. [DOI] [PubMed] [Google Scholar]
- Reuser A. J., Kroos M., Oude Elferink R. P., Tager J. M. Defects in synthesis, phosphorylation, and maturation of acid alpha-glucosidase in glycogenosis type II. J Biol Chem. 1985 Jul 15;260(14):8336–8341. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Salafsky I. S., Nadler H. L. A fluorometric assay of alpha-glucosidase and its application in the study of Pompe's disease. J Lab Clin Med. 1973 Mar;81(3):450–454. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Swallow D. M., Corney G., Harris H., Hirschhorn R. Acid alpha-glucosidase: a new polymorphism in man demonstrable by 'affinity' electrophoresis. Ann Hum Genet. 1975 May;38(4):391–406. doi: 10.1111/j.1469-1809.1975.tb00629.x. [DOI] [PubMed] [Google Scholar]
- Tager J. M., Oude Elferink R. P., Reuser A., Kroos M., Ginsel L. A., Fransen J. A., Klumperman J. alpha-Glucosidase deficiency (Pompe's disease). Enzyme. 1987;38(1-4):280–285. doi: 10.1159/000469217. [DOI] [PubMed] [Google Scholar]
- Tzall S., Martiniuk F., Adler A., Hirschhorn R. Identification of an RsaI RFLP at the acid alpha glucosidase (GAA) locus. Nucleic Acids Res. 1990 Mar 25;18(6):1661–1661. doi: 10.1093/nar/18.6.1661-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzall S., Martiniuk F., Hirschhorn R. Further characterization of SacI RFLPs at the acid alpha glucosidase (GAA) locus. Nucleic Acids Res. 1990 Apr 11;18(7):1930–1930. doi: 10.1093/nar/18.7.1930-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg A. T., Hoefsloot L. H., Hoogeveen-Westerveld M., Petersen E. M., Reuser A. J. Glycogenosis type II: protein and DNA analysis in five South African families from various ethnic origins. Am J Hum Genet. 1989 Jun;44(6):787–793. [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]