Abstract
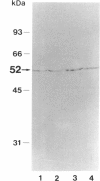
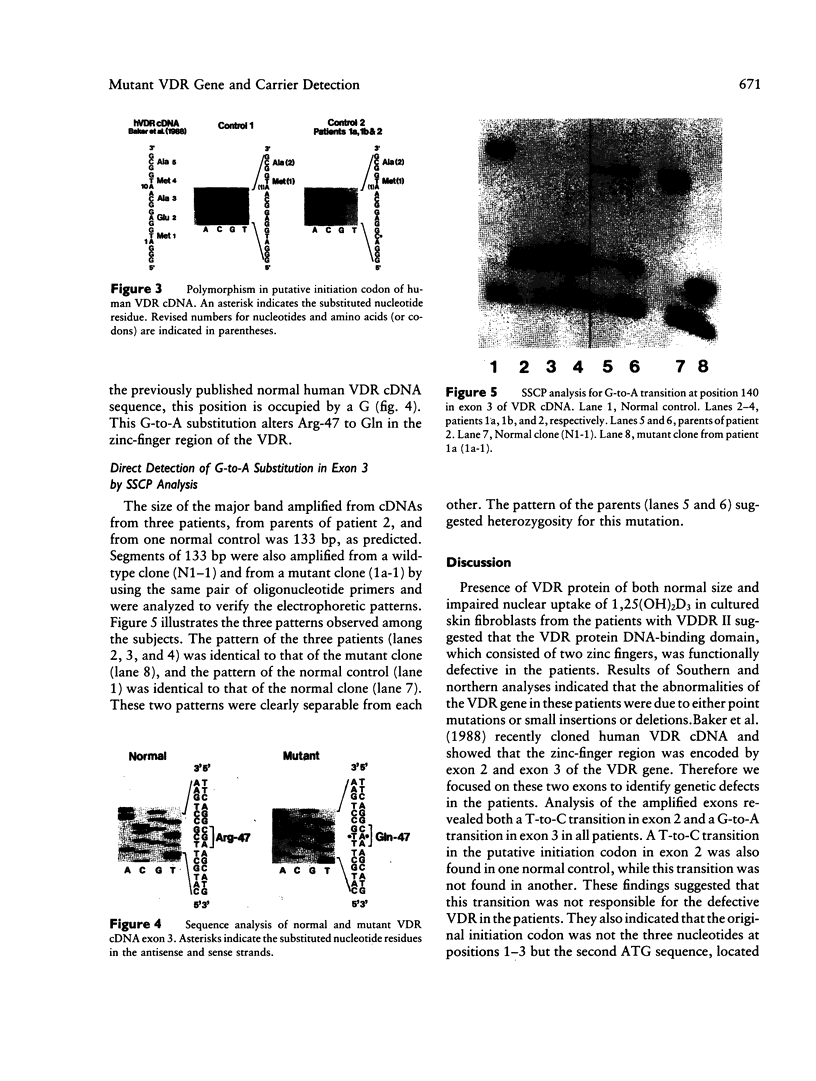
Vitamin D-dependent rickets type II is a hereditary disease resulting from a defective vitamin D receptor. In three Japanese patients with vitamin D-dependent rickets type II whose fibroblasts displayed normal cytosol binding and impaired nuclear uptake of 1,25-dihydroxyvitamin D3, western, Southern, and northern analyses failed to disclose any abnormalities in vitamin D3 receptor protein and its gene. Exons 2 and 3 of the vitamin D receptor cDNA, which encode the DNA-binding domain consisting of two zinc fingers, were amplified by PCR and sequenced to identify the specific mutations in the vitamin D receptor gene. In the three patients and one normal control a T-to-C transition was found in the putative initiation codon, while this transition was not observed in another normal control. This finding suggested that an original initiation codon was located at positions 10-12 in the human vitamin D receptor cDNA sequence reported previously. In contrast, a unique G-to-A transition at position 140 in exon 3, resulting in substitution of arginine by glutamine at residue 47, was revealed only in these three patients. The arginine at 47 is located between two zinc fingers and is conserved within all steroid hormone receptors. Therefore, it is highly conceivable that this amino acid substitution is responsible for the defect of the vitamin D receptor in the patients. Single-strand conformation polymorphism analysis of amplified DNA confirmed that all patients were homozygous and that parents from one family were heterozygous carriers for this mutation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker A. R., McDonnell D. P., Hughes M., Crisp T. M., Mangelsdorf D. J., Haussler M. R., Pike J. W., Shine J., O'Malley B. W. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci U S A. 1988 May;85(10):3294–3298. doi: 10.1073/pnas.85.10.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Hata A., Robertson M., Emi M., Lalouel J. M. Direct detection and automated sequencing of individual alleles after electrophoretic strand separation: identification of a common nonsense mutation in exon 9 of the human lipoprotein lipase gene. Nucleic Acids Res. 1990 Sep 25;18(18):5407–5411. doi: 10.1093/nar/18.18.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M. R., Malloy P. J., Kieback D. G., Kesterson R. A., Pike J. W., Feldman D., O'Malley B. W. Point mutations in the human vitamin D receptor gene associated with hypocalcemic rickets. Science. 1988 Dec 23;242(4886):1702–1705. doi: 10.1126/science.2849209. [DOI] [PubMed] [Google Scholar]
- Kerner S. A., Scott R. A., Pike J. W. Sequence elements in the human osteocalcin gene confer basal activation and inducible response to hormonal vitamin D3. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4455–4459. doi: 10.1073/pnas.86.12.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman U. A., Eil C., Marx S. J. Resistance to 1,25-dihydroxyvitamin D. Association with heterogeneous defects in cultured skin fibroblasts. J Clin Invest. 1983 Feb;71(2):192–200. doi: 10.1172/JCI110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy P. J., Hochberg Z., Pike J. W., Feldman D. Abnormal binding of vitamin D receptors to deoxyribonucleic acid in a kindred with vitamin D-dependent rickets, type II. J Clin Endocrinol Metab. 1989 Feb;68(2):263–269. doi: 10.1210/jcem-68-2-263. [DOI] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Pike J. W. Monoclonal antibodies to chick intestinal receptors for 1,25-dihydroxyvitamin D3. Interaction and effects of binding on receptor function. J Biol Chem. 1984 Jan 25;259(2):1167–1173. [PubMed] [Google Scholar]
- Sone T., Marx S. J., Liberman U. A., Pike J. W. A unique point mutation in the human vitamin D receptor chromosomal gene confers hereditary resistance to 1,25-dihydroxyvitamin D3. Mol Endocrinol. 1990 Apr;4(4):623–631. doi: 10.1210/mend-4-4-623. [DOI] [PubMed] [Google Scholar]
- Takeda E., Kuroda Y., Saijo T., Toshima K., Naito E., Kobashi H., Iwakuni Y., Miyao M. Rapid diagnosis of vitamin D-dependent rickets type II by use of phytohemagglutinin-stimulated lymphocytes. Clin Chim Acta. 1986 Mar 28;155(3):245–250. doi: 10.1016/0009-8981(86)90244-5. [DOI] [PubMed] [Google Scholar]
- Takeda E., Yokota I., Ito M., Kobashi H., Saijo T., Kuroda Y. 25-Hydroxyvitamin D-24-hydroxylase in phytohemagglutinin-stimulated lymphocytes: intermediate bioresponse to 1,25-dihydroxyvitamin D3 of cells from parents of patients with vitamin D-dependent rickets type II. J Clin Endocrinol Metab. 1990 Apr;70(4):1068–1074. doi: 10.1210/jcem-70-4-1068. [DOI] [PubMed] [Google Scholar]
- Takeda E., Yokota I., Kawakami I., Hashimoto T., Kuroda Y., Arase S. Two siblings with vitamin-D-dependent rickets type II: no recurrence of rickets for 14 years after cessation of therapy. Eur J Pediatr. 1989 Oct;149(1):54–57. doi: 10.1007/BF02024336. [DOI] [PubMed] [Google Scholar]