Abstract
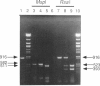
The second enzyme in the heme biosynthetic pathway, delta-aminolevulinate dehydratase (ALAD), is a homooctameric protein encoded by a gene localized to human chromosome 9q34. Expression of the two common alleles, ALAD1 (p = .9) and ALAD2 (q = .1), results in a polymorphic enzyme system with three distinct charge isozymes, designated 1-1, 1-2, and 2-2. Individuals heterozygous (2pq = .18) or homozygous (q2 = .01) for the ALAD2 allele have significantly higher blood lead levels than do ALAD1 homozygotes, when exposed to low or high levels of lead in the environment. To investigate the molecular nature of this common polymorphism, total RNA from an ALAD2 homozygote was oligo-dT primed and reverse transcribed, and then the ALAD2 cDNA was amplified, subcloned, and sequenced. Compared with the ALAD1 sequence, the only difference in the ALAD2 cDNA was a G-to-C transversion of nucleotide 177 in the coding region, which created an MspI restriction site. This base substitution predicted the replacement of a positively charged lysine by a neutral asparagine (K59N), an amino acid change consistent with the more electronegative charge of the ALAD-2 subunit. The ALAD1 and ALAD2 alleles were easily detected by amplification of a 916-bp region of genomic DNA and MspI digestion which results in 582- and 511-bp products, respectively. Molecular analysis of 85 ALAD1/ALAD2 heterozygotes and of eight ALAD2 homozygotes revealed no discrepancy between the predicted genotype and the erythrocyte isozyme phenotype, indicating that all the ALAD2 alleles analyzed had the G-to-C transversion.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Desnick R. J. Purification and properties of delta-aminolevulinate dehydrase from human erythrocytes. J Biol Chem. 1979 Aug 10;254(15):6924–6930. [PubMed] [Google Scholar]
- Astrin K. H., Bishop D. F., Wetmur J. G., Kaul B., Davidow B., Desnick R. J. delta-Aminolevulinic acid dehydratase isozymes and lead toxicity. Ann N Y Acad Sci. 1987;514:23–29. doi: 10.1111/j.1749-6632.1987.tb48757.x. [DOI] [PubMed] [Google Scholar]
- Audesirk G. Effects of lead exposure on the physiology of neurons. Prog Neurobiol. 1985;24(3):199–231. doi: 10.1016/0301-0082(85)90006-1. [DOI] [PubMed] [Google Scholar]
- Battistuzzi G., Petrucci R., Silvagni L., Urbani F. R., Caiola S. delta-Aminolevulinate dehydrase: a new genetic polymorphism in man. Ann Hum Genet. 1981 Jul;45(Pt 3):223–229. doi: 10.1111/j.1469-1809.1981.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Bellinger D., Leviton A., Waternaux C., Needleman H., Rabinowitz M. Longitudinal analyses of prenatal and postnatal lead exposure and early cognitive development. N Engl J Med. 1987 Apr 23;316(17):1037–1043. doi: 10.1056/NEJM198704233161701. [DOI] [PubMed] [Google Scholar]
- Benkmann H. G., Bogdanski P., Goedde H. W. Polymorphism of delta-aminolevulinic acid dehydratase in various populations. Hum Hered. 1983;33(1):62–64. doi: 10.1159/000153351. [DOI] [PubMed] [Google Scholar]
- Bevan D. R., Bodlaender P., Shemin D. Mechanism of porphobilinogen synthase. Requirement of Zn2+ for enzyme activity. J Biol Chem. 1980 Mar 10;255(5):2030–2035. [PubMed] [Google Scholar]
- Bishop T. R., Frelin L. P., Boyer S. H. Nucleotide sequence of rat liver delta-aminolevulinic acid dehydratase cDNA. Nucleic Acids Res. 1986 Dec 22;14(24):10115–10115. doi: 10.1093/nar/14.24.10115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop T. R., Hodes Z. I., Frelin L. P., Boyer S. H. Cloning and sequence of mouse erythroid delta-aminolevulinate dehydratase cDNA. Nucleic Acids Res. 1989 Feb 25;17(4):1775–1775. doi: 10.1093/nar/17.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Bottomley S. S., Muller-Eberhard U. Pathophysiology of heme synthesis. Semin Hematol. 1988 Oct;25(4):282–302. [PubMed] [Google Scholar]
- Brennan M. J., Cantrill R. C. Delta-aminolaevulinic acid is a potent agonist for GABA autoreceptors. Nature. 1979 Aug 9;280(5722):514–515. doi: 10.1038/280514a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cutler M. G., McLaughlin M., McNeil E., Moore M. R. Effects of delta-aminolaevulinic acid on contractile activity in the isolated small intestine of the rabbit. Role of adrenergic receptors. Neuropharmacology. 1985 Oct;24(10):1005–1009. doi: 10.1016/0028-3908(85)90129-7. [DOI] [PubMed] [Google Scholar]
- Dent A. J., Beyersmann D., Block C., Hasnain S. S. Two different zinc sites in bovine 5-aminolevulinate dehydratase distinguished by extended X-ray absorption fine structure. Biochemistry. 1990 Aug 28;29(34):7822–7828. doi: 10.1021/bi00486a007. [DOI] [PubMed] [Google Scholar]
- Doss M., Becker U., Sixel F., Geisse S., Solcher H., Schneider J., Kufner G., Schlegel H., Stoeppler M. Persistent protoporphyrinemia in hereditary porphobilinogen synthase (delta-aminolevulinic acid dehydrase) deficiency under low lead exposure. A new molecular basis for the pathogenesis of lead intoxication. Klin Wochenschr. 1982 Jun 15;60(12):599–606. doi: 10.1007/BF01711435. [DOI] [PubMed] [Google Scholar]
- Doss M., Laubenthal F., Stoeppler M. Lead poisoning in inherited delta-aminolevulinic acid dehydratase deficiency. Int Arch Occup Environ Health. 1984;54(1):55–63. doi: 10.1007/BF00378728. [DOI] [PubMed] [Google Scholar]
- Doss M., von Tiepermann R., Schneider J., Schmid H. New type of hepatic porphyria with porphobilinogen synthase defect and intermittent acute clinical manifestation. Klin Wochenschr. 1979 Oct 15;57(20):1123–1127. doi: 10.1007/BF01481493. [DOI] [PubMed] [Google Scholar]
- Echelard Y., Dymetryszyn J., Drolet M., Sasarman A. Nucleotide sequence of the hemB gene of Escherichia coli K12. Mol Gen Genet. 1988 Nov;214(3):503–508. doi: 10.1007/BF00330487. [DOI] [PubMed] [Google Scholar]
- Geisse S., Brüller H. J., Doss M. Porphobilinogen synthase (delta-aminolevulinic acid dehydratase) activity in human erythrocytes: reactivation by zinc and dithiothreitol depending on influence of storage. Clin Chim Acta. 1983 Dec 15;135(2):239–245. doi: 10.1016/0009-8981(83)90141-9. [DOI] [PubMed] [Google Scholar]
- Jaffe E. K., Salowe S. P., Chen N. T., DeHaven P. A. Porphobilinogen synthase modification with methylmethanethiosulfonate. A protocol for the investigation of metalloproteins. J Biol Chem. 1984 Apr 25;259(8):5032–5036. [PubMed] [Google Scholar]
- Minnema D. J., Michaelson I. A. Differential effects of inorganic lead and delta-aminolevulinic acid in vitro on synaptosomal gamma-aminobutyric acid release. Toxicol Appl Pharmacol. 1986 Dec;86(3):437–447. doi: 10.1016/0041-008x(86)90371-6. [DOI] [PubMed] [Google Scholar]
- Müller W. E., Snyder S. H. delta-Aminolevulinic acid: influences on synaptic GABA receptor binding may explain CNS symptoms of porphyria. Ann Neurol. 1977 Oct;2(4):340–342. doi: 10.1002/ana.410020415. [DOI] [PubMed] [Google Scholar]
- Needleman H. L., Schell A., Bellinger D., Leviton A., Allred E. N. The long-term effects of exposure to low doses of lead in childhood. An 11-year follow-up report. N Engl J Med. 1990 Jan 11;322(2):83–88. doi: 10.1056/NEJM199001113220203. [DOI] [PubMed] [Google Scholar]
- Petrucci R., Leonardi A., Battistuzzi G. The genetic polymorphism of delta-aminolevulinate dehydrase in Italy. Hum Genet. 1982;60(3):289–290. doi: 10.1007/BF00303023. [DOI] [PubMed] [Google Scholar]
- Potluri V. R., Astrin K. H., Wetmur J. G., Bishop D. F., Desnick R. J. Human delta-aminolevulinate dehydratase: chromosomal localization to 9q34 by in situ hybridization. Hum Genet. 1987 Jul;76(3):236–239. doi: 10.1007/BF00283614. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F. Determination of nucleotide sequences in DNA. Science. 1981 Dec 11;214(4526):1205–1210. doi: 10.1126/science.7302589. [DOI] [PubMed] [Google Scholar]
- Schlösser M., Beyersmann D. Zinc and cadmium 5-aminolevulinate dehydratase. Metal-dependent pH profiles. Biol Chem Hoppe Seyler. 1987 Nov;368(11):1469–1477. doi: 10.1515/bchm3.1987.368.2.1469. [DOI] [PubMed] [Google Scholar]
- Tsukamoto I., Yoshinaga T., Sano S. The role of zinc with special reference to the essential thiol groups in delta-aminolevulinic acid dehydratase of bovine liver. Biochim Biophys Acta. 1979 Sep 12;570(1):167–178. doi: 10.1016/0005-2744(79)90211-0. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Bishop D. F., Cantelmo C., Desnick R. J. Human delta-aminolevulinate dehydratase: nucleotide sequence of a full-length cDNA clone. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7703–7707. doi: 10.1073/pnas.83.20.7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W. H., Shemin D., Richards K. E., Williams R. C. The quaternary structure of delta-aminolevulinic acid dehydratase from bovine liver. Proc Natl Acad Sci U S A. 1974 May;71(5):1767–1770. doi: 10.1073/pnas.71.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemsen B., Angerer J., Lehnert G., Benkmann H. G., Goedde H. W. Polymorphism of delta-aminolevulinic acid dehydratase in lead-exposed workers. Int Arch Occup Environ Health. 1986;58(3):245–247. doi: 10.1007/BF00432107. [DOI] [PubMed] [Google Scholar]