Abstract
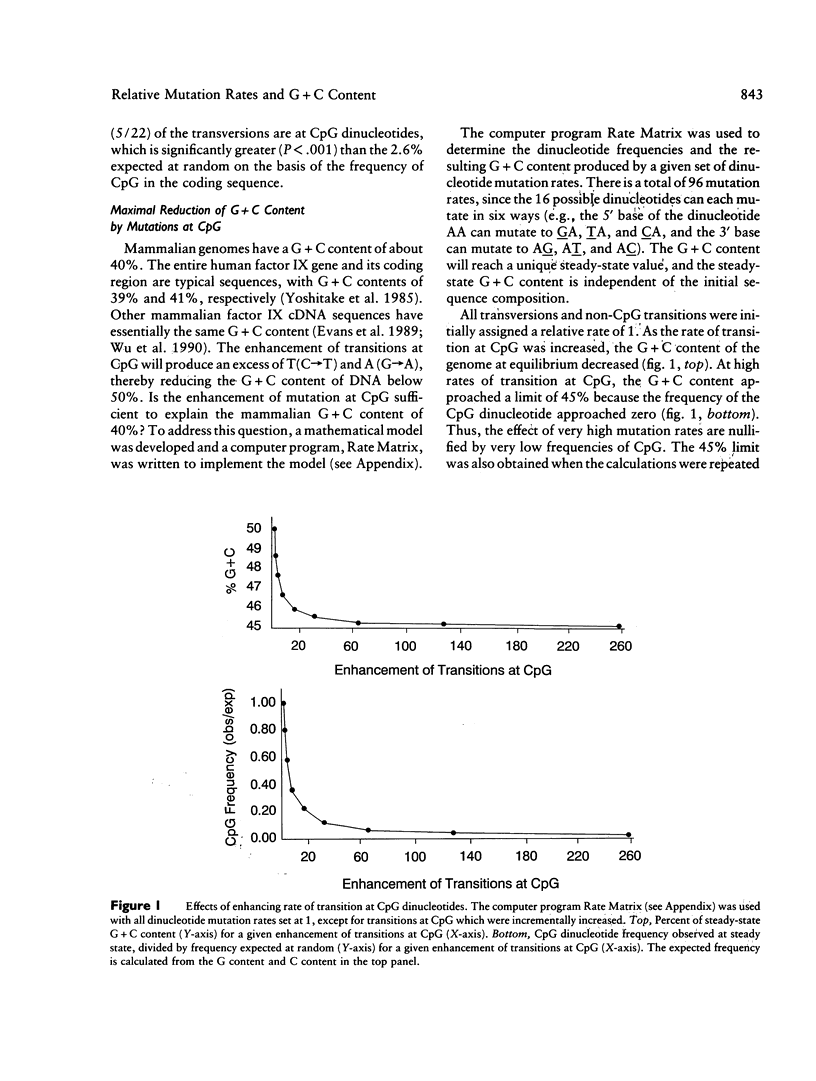
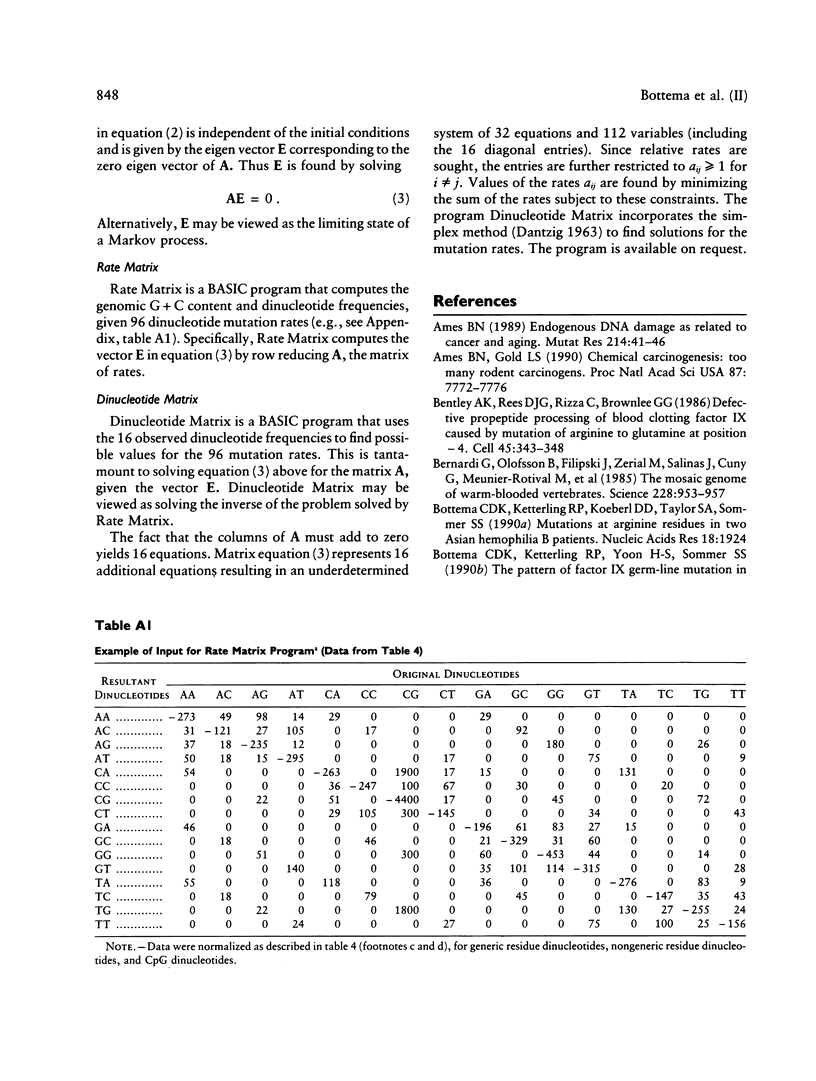
The factor IX gene has a G + C content of approximately 40% in all mammalian species examined. In human factor IX, C----T and G----A transitions at the dinucleotide CpG are elevated at least 24-fold relative to other transitions. Can the G + C content be explained solely by this hot spot of mutation? Using our mathematical model, we show that the elevation of mutation at CpG cannot alone lower the G + C content below 45%. To search for other hot spots of mutation that might contribute to the reduction of G + C content, we assessed the relative rates of base substitution in our sample of 160 families with hemophilia B. Seventeen independent single-base substitutions are reported herein for a total of 96 independent point mutations in our sample. The following conclusions emerge from the analysis of our data and, where appropriate, the data of others: (1) Transversions at CpG are elevated an estimated 7.7-fold relative to other transversions. (2) The mutation rates at non-CpG dinucleotides are remarkably uniform; none of the observed rates are either more than twofold above the median for transitions or more than threefold above the median for transversions. (3) The pattern of recent mutation is compatible with the pattern during mammalian evolution that has maintained the G + C content of the factor IX gene at approximately 40%.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Endogenous DNA damage as related to cancer and aging. Mutat Res. 1989 Sep;214(1):41–46. doi: 10.1016/0027-5107(89)90196-6. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Gold L. S. Chemical carcinogenesis: too many rodent carcinogens. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7772–7776. doi: 10.1073/pnas.87.19.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley A. K., Rees D. J., Rizza C., Brownlee G. G. Defective propeptide processing of blood clotting factor IX caused by mutation of arginine to glutamine at position -4. Cell. 1986 May 9;45(3):343–348. doi: 10.1016/0092-8674(86)90319-3. [DOI] [PubMed] [Google Scholar]
- Bernardi G., Olofsson B., Filipski J., Zerial M., Salinas J., Cuny G., Meunier-Rotival M., Rodier F. The mosaic genome of warm-blooded vertebrates. Science. 1985 May 24;228(4702):953–958. doi: 10.1126/science.4001930. [DOI] [PubMed] [Google Scholar]
- Bottema C. D., Ketterling R. P., Ii S., Yoon H. S., Phillips J. A., 3rd, Sommer S. S. Missense mutations and evolutionary conservation of amino acids: evidence that many of the amino acids in factor IX function as "spacer" elements. Am J Hum Genet. 1991 Oct;49(4):820–838. [PMC free article] [PubMed] [Google Scholar]
- Bottema C. D., Ketterling R. P., Koeberl D. D., Taylor S. A., Sommer S. S. Mutations at arginine residues in two Asian hemophilia B patients. Nucleic Acids Res. 1990 Apr 11;18(7):1924–1924. doi: 10.1093/nar/18.7.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottema C. D., Ketterling R. P., Yoon H. S., Sommer S. S. The pattern of factor IX germ-line mutation in Asians is similar to that of Caucasians. Am J Hum Genet. 1990 Nov;47(5):835–841. [PMC free article] [PubMed] [Google Scholar]
- Bottema C. D., Koeberl D. D., Ketterling R. P., Bowie E. J., Taylor S. A., Lillicrap D., Shapiro A., Gilchrist G., Sommer S. S. A past mutation at isoleucine 397 is now a common cause of moderate/mild haemophilia B. Br J Haematol. 1990 Jun;75(2):212–216. doi: 10.1111/j.1365-2141.1990.tb02651.x. [DOI] [PubMed] [Google Scholar]
- Camerino G., Grzeschik K. H., Jaye M., De La Salle H., Tolstoshev P., Lecocq J. P., Heilig R., Mandel J. L. Regional localization on the human X chromosome and polymorphism of the coagulation factor IX gene (hemophilia B locus). Proc Natl Acad Sci U S A. 1984 Jan;81(2):498–502. doi: 10.1073/pnas.81.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R., Neel J. V. Description and validation of a method for simultaneous estimation of effective population size and mutation rate from human population data. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9407–9411. doi: 10.1073/pnas.86.23.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diuguid D. L., Rabiet M. J., Furie B. C., Furie B. Molecular defects of factor IX Chicago-2 (Arg 145----His) and prothrombin Madrid (Arg 271----cys): arginine mutations that preclude zymogen activation. Blood. 1989 Jul;74(1):193–200. [PubMed] [Google Scholar]
- Evans J. P., Watzke H. H., Ware J. L., Stafford D. W., High K. A. Molecular cloning of a cDNA encoding canine factor IX. Blood. 1989 Jul;74(1):207–212. [PubMed] [Google Scholar]
- Eyster M. E., Lewis J. H., Shapiro S. S., Gill F., Kajani M., Prager D., Djerassi I., Rice S., Lusch C., Keller A. The Pennsylvania hemophilia program 1973-1978. Am J Hematol. 1980;9(3):277–286. doi: 10.1002/ajh.2830090306. [DOI] [PubMed] [Google Scholar]
- Giannelli F., Green P. M., High K. A., Lozier J. N., Lillicrap D. P., Ludwig M., Olek K., Reitsma P. H., Goossens M., Yoshioka A. Haemophilia B: database of point mutations and short additions and deletions. Nucleic Acids Res. 1990 Jul 25;18(14):4053–4059. doi: 10.1093/nar/18.14.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. M., Bentley D. R., Mibashan R. S., Nilsson I. M., Giannelli F. Molecular pathology of haemophilia B. EMBO J. 1989 Apr;8(4):1067–1072. doi: 10.1002/j.1460-2075.1989.tb03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. M., Montandon A. J., Bentley D. R., Ljung R., Nilsson I. M., Giannelli F. The incidence and distribution of CpG----TpG transitions in the coagulation factor IX gene. A fresh look at CpG mutational hotspots. Nucleic Acids Res. 1990 Jun 11;18(11):3227–3231. doi: 10.1093/nar/18.11.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson S., Proper J. A., Bowie E. J., Sommer S. S. Parameters affecting the yield of DNA from human blood. Anal Biochem. 1987 Sep;165(2):294–299. doi: 10.1016/0003-2697(87)90272-7. [DOI] [PubMed] [Google Scholar]
- Ketterling R. P., Bottema C. D., Phillips J. A., 3rd, Sommer S. S. Evidence that descendants of three founders constitute about 25% of hemophilia B in the United States. Genomics. 1991 Aug;10(4):1093–1096. doi: 10.1016/0888-7543(91)90207-u. [DOI] [PubMed] [Google Scholar]
- Koeberl D. D., Bottema C. D., Buerstedde J. M., Sommer S. S. Functionally important regions of the factor IX gene have a low rate of polymorphism and a high rate of mutation in the dinucleotide CpG. Am J Hum Genet. 1989 Sep;45(3):448–457. [PMC free article] [PubMed] [Google Scholar]
- Koeberl D. D., Bottema C. D., Ketterling R. P., Bridge P. J., Lillicrap D. P., Sommer S. S. Mutations causing hemophilia B: direct estimate of the underlying rates of spontaneous germ-line transitions, transversions, and deletions in a human gene. Am J Hum Genet. 1990 Aug;47(2):202–217. [PMC free article] [PubMed] [Google Scholar]
- Liddell M. B., Lillicrap D. P., Peake I. R., Bloom A. L. Defective propeptide processing and abnormal activation underlie the molecular pathology of factor IX Troed-y-Rhiw. Br J Haematol. 1989 Jun;72(2):208–215. doi: 10.1111/j.1365-2141.1989.tb07684.x. [DOI] [PubMed] [Google Scholar]
- Loeb L. A. Endogenous carcinogenesis: molecular oncology into the twenty-first century--presidential address. Cancer Res. 1989 Oct 15;49(20):5489–5496. [PubMed] [Google Scholar]
- Mandel J. L. Dystrophin. The gene and its product. Nature. 1989 Jun 22;339(6226):584–586. doi: 10.1038/339584a0. [DOI] [PubMed] [Google Scholar]
- Montandon A. J., Green P. M., Bentley D. R., Ljung R., Nilsson I. M., Giannelli F. Two factor IX mutations in the family of an isolated haemophilia B patient: direct carrier diagnosis by amplification mismatch detection (AMD). Hum Genet. 1990 Jul;85(2):200–204. doi: 10.1007/BF00193196. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes C. M., Griffith M. J., Roberts H. R., Lundblad R. L. Identification of the molecular defect in factor IX Chapel Hill: substitution of histidine for arginine at position 145. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4200–4202. doi: 10.1073/pnas.80.14.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussinov R. Eukaryotic dinucleotide preference rules and their implications for degenerate codon usage. J Mol Biol. 1981 Jun 15;149(1):125–131. doi: 10.1016/0022-2836(81)90264-3. [DOI] [PubMed] [Google Scholar]
- SWARTZ M. N., TRAUTNER T. A., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. XI. Further studies on nearest neighbor base sequences in deoxyribonucleic acids. J Biol Chem. 1962 Jun;237:1961–1967. [PubMed] [Google Scholar]
- Smith C. W., Porro E. B., Patton J. G., Nadal-Ginard B. Scanning from an independently specified branch point defines the 3' splice site of mammalian introns. Nature. 1989 Nov 16;342(6247):243–247. doi: 10.1038/342243a0. [DOI] [PubMed] [Google Scholar]
- Sommer S. S. Mutagen test. Nature. 1990 Jul 5;346(6279):22–23. doi: 10.1038/346022b0. [DOI] [PubMed] [Google Scholar]
- Sugimoto M., Miyata T., Kawabata S., Yoshioka A., Fukui H., Iwanaga S. Factor IX Kawachinagano: impaired function of the Gla-domain caused by attached propeptide region due to substitution of arginine by glutamine at position -4. Br J Haematol. 1989 Jun;72(2):216–221. doi: 10.1111/j.1365-2141.1989.tb07685.x. [DOI] [PubMed] [Google Scholar]
- Sved J., Bird A. The expected equilibrium of the CpG dinucleotide in vertebrate genomes under a mutation model. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4692–4696. doi: 10.1073/pnas.87.12.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. R., Bajaj S. P., Chen S. H., MacGillivray R. T. "Founder" effect in different families with haemophilia B mutation. Lancet. 1990 Feb 17;335(8686):418–418. doi: 10.1016/0140-6736(90)90259-8. [DOI] [PubMed] [Google Scholar]
- Wang N. S., Chen S. H., Thompson A. R. Point mutations in four hemophilia B patients from China. Thromb Haemost. 1990 Oct 22;64(2):302–306. [PubMed] [Google Scholar]
- Ware J., Diuguid D. L., Liebman H. A., Rabiet M. J., Kasper C. K., Furie B. C., Furie B., Stafford D. W. Factor IX San Dimas. Substitution of glutamine for Arg-4 in the propeptide leads to incomplete gamma-carboxylation and altered phospholipid binding properties. J Biol Chem. 1989 Jul 5;264(19):11401–11406. [PubMed] [Google Scholar]
- Wiebauer K., Jiricny J. In vitro correction of G.T mispairs to G.C pairs in nuclear extracts from human cells. Nature. 1989 May 18;339(6221):234–236. doi: 10.1038/339234a0. [DOI] [PubMed] [Google Scholar]
- Winship P. R., Anson D. S., Rizza C. R., Brownlee G. G. Carrier detection in haemophilia B using two further intragenic restriction fragment length polymorphisms. Nucleic Acids Res. 1984 Dec 11;12(23):8861–8872. doi: 10.1093/nar/12.23.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship P. R., Rees D. J., Alkan M. Detection of polymorphisms at cytosine phosphoguanadine dinucleotides and diagnosis of haemophilia B carriers. Lancet. 1989 Mar 25;1(8639):631–634. doi: 10.1016/s0140-6736(89)92141-7. [DOI] [PubMed] [Google Scholar]



