Abstract
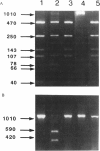
Monoamine oxidase (MAO) is a critical enzyme in the degradative deamination of biogenic amines throughout the body. Two biochemically distinct forms of the enzyme, A and B, are encoded in separate genes on the human X chromosome. In these studies we investigated the role of the structural gene for MAO-A in determining levels of activity in humans, as measured in cultured skin fibroblasts. The coding sequence of the mRNA for MAO-A was determined by first-strand cDNA synthesis, PCR amplification, and direct dideoxy sequencing. Two single-basepair substitutions were observed in cDNAs from cells with a 30-fold difference in activity levels. These two substitutions were in the third base of a triplet codon and hence did not affect the deduced amino acid sequence but did affect the presence or absence of restriction-enzyme sites for EcoRV and Fnu4HI, which could be elucidated on PCR fragments derived from genomic DNA or cDNAs. A third polymorphism for MspI in the noncoding region of the MAOA gene was also evaluated by Southern blot analysis using genomic DNA. Statistically significant associations were observed between the alleles for MAOA and levels of MAO activity in human male fibroblast lines. This association indicates that the MAOA gene itself is a major determinant of activity levels, apparently, in part, through noncoding, regulatory elements.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach A. W., Lan N. C., Johnson D. L., Abell C. W., Bembenek M. E., Kwan S. W., Seeburg P. H., Shih J. C. cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4934–4938. doi: 10.1073/pnas.85.13.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond P. A., Cundall R. L. Properties of monoamine oxidase (MAO) in human blood platelets, plasma, lymphocytes and granulocytes. Clin Chim Acta. 1977 Oct 15;80(2):317–326. doi: 10.1016/0009-8981(77)90039-0. [DOI] [PubMed] [Google Scholar]
- Breakefield X. O., Castiglione C. M., Edelstein S. B. Monoamine oxidase activity decreased in cells lacking hypoxanthine phosphoribosyltransferase activity. Science. 1976 Jun 4;192(4243):1018–1020. doi: 10.1126/science.1273584. [DOI] [PubMed] [Google Scholar]
- Breakefield X. O., Giller E. L., Jr, Nurnberger J. I., Jr, Castiglione C. M., Buchsbaum M. S., Gershon E. S. Monoamine oxidase type A in fibroblasts from patients with bipolar depressive illness. Psychiatry Res. 1980 Jul;2(3):307–314. doi: 10.1016/0165-1781(80)90022-0. [DOI] [PubMed] [Google Scholar]
- Campbell I. C., Robinson D. S., Lovenberg W., Murphy D. L. The effects of chronic regimens of clorgyline and pargyline on monoamine metabolism in the rat brain. J Neurochem. 1979 Jan;32(1):49–55. doi: 10.1111/j.1471-4159.1979.tb04508.x. [DOI] [PubMed] [Google Scholar]
- Castro Costa M. R., Edelstein S. B., Castiglione C. M., Chao H., Breakefield X. O. Properties of monoamine oxidase in control and Lesch-Nyhan fibroblasts. Biochem Genet. 1980 Jun;18(5-6):577–590. doi: 10.1007/BF00484403. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Diergaarde P. J., Wieringa B., Bleeker-Wagemakers E. M., Sims K. B., Breakefield X. O., Ropers H. H. Physical fine-mapping of a deletion spanning the Norrie gene. Hum Genet. 1989 Dec;84(1):22–26. doi: 10.1007/BF00210665. [DOI] [PubMed] [Google Scholar]
- Donnai D., Mountford R. C., Read A. P. Norrie disease resulting from a gene deletion: clinical features and DNA studies. J Med Genet. 1988 Feb;25(2):73–78. doi: 10.1136/jmg.25.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly C. H., Murphy D. L. Substrate- and inhibitor-related characteristics of human platelet monoamine oxidase. Biochem Pharmacol. 1977 May 1;26(9):853–858. doi: 10.1016/0006-2952(77)90398-7. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Edelstein S. B., Breakefield X. O. Monoamine oxidases A and B are differentially regulated by glucocorticoids and "aging" in human skin fibroblasts. Cell Mol Neurobiol. 1986 Jun;6(2):121–150. doi: 10.1007/BF00711066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein S. B., Castiglione C. M., Breakfield X. O. Monoamine oxidase activity in normal and Lesch-Nyhan fibroblasts. J Neurochem. 1978 Nov;31(5):1247–1254. doi: 10.1111/j.1471-4159.1978.tb06249.x. [DOI] [PubMed] [Google Scholar]
- Fowler J. S., MacGregor R. R., Wolf A. P., Arnett C. D., Dewey S. L., Schlyer D., Christman D., Logan J., Smith M., Sachs H. Mapping human brain monoamine oxidase A and B with 11C-labeled suicide inactivators and PET. Science. 1987 Jan 23;235(4787):481–485. doi: 10.1126/science.3099392. [DOI] [PubMed] [Google Scholar]
- Gal A., Wieringa B., Smeets D. F., Bleeker-Wagemakers L., Ropers H. H. Submicroscopic interstitial deletion of the X chromosome explains a complex genetic syndrome dominated by Norrie disease. Cytogenet Cell Genet. 1986;42(4):219–224. doi: 10.1159/000132282. [DOI] [PubMed] [Google Scholar]
- Garrick N. A., Murphy D. L. Monoamine oxidase type A: differences in selectivity towards l-norepinephrine compared to serotonin. Biochem Pharmacol. 1982 Dec 15;31(24):4061–4066. doi: 10.1016/0006-2952(82)90656-6. [DOI] [PubMed] [Google Scholar]
- Gorman K. B., Steinberg R. A. Simplified method for selective amplification and direct sequencing of cDNAs. Biotechniques. 1989 Apr;7(4):326-8, 331. [PubMed] [Google Scholar]
- Hsu Y. P., Powell J. F., Sims K. B., Breakefield X. O. Molecular genetics of the monoamine oxidases. J Neurochem. 1989 Jul;53(1):12–18. doi: 10.1111/j.1471-4159.1989.tb07289.x. [DOI] [PubMed] [Google Scholar]
- Hsu Y. P., Weyler W., Chen S., Sims K. B., Rinehart W. B., Utterback M. C., Powell J. F., Breakefield X. O. Structural features of human monoamine oxidase A elucidated from cDNA and peptide sequences. J Neurochem. 1988 Oct;51(4):1321–1324. doi: 10.1111/j.1471-4159.1988.tb03105.x. [DOI] [PubMed] [Google Scholar]
- Lan N. C., Heinzmann C., Gal A., Klisak I., Orth U., Lai E., Grimsby J., Sparkes R. S., Mohandas T., Shih J. C. Human monoamine oxidase A and B genes map to Xp 11.23 and are deleted in a patient with Norrie disease. Genomics. 1989 May;4(4):552–559. doi: 10.1016/0888-7543(89)90279-6. [DOI] [PubMed] [Google Scholar]
- Levitt P., Pintar J. E., Breakefield X. O. Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6385–6389. doi: 10.1073/pnas.79.20.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy E. R., Powell J. F., Buckle V. J., Hsu Y. P., Breakefield X. O., Craig I. W. Localization of human monoamine oxidase-A gene to Xp11.23-11.4 by in situ hybridization: implications for Norrie disease. Genomics. 1989 Aug;5(2):368–370. doi: 10.1016/0888-7543(89)90072-4. [DOI] [PubMed] [Google Scholar]
- Murphy D. L., Kalin N. H. Biological and behavioral consequences of alterations in monoamine oxidase activity. Schizophr Bull. 1980;6(2):355–367. doi: 10.1093/schbul/6.2.355. [DOI] [PubMed] [Google Scholar]
- Murphy D. L., Sims K. B., Karoum F., Garrick N. A., de la Chapelle A., Sankila E. M., Norio R., Breakefield X. O. Plasma amine oxidase activities in Norrie disease patients with an X-chromosomal deletion affecting monoamine oxidase. J Neural Transm Gen Sect. 1991;83(1-2):1–12. doi: 10.1007/BF01244447. [DOI] [PubMed] [Google Scholar]
- Murphy D. L., Sims K. B., Karoum F., de la Chapelle A., Norio R., Sankila E. M., Breakefield X. O. Marked amine and amine metabolite changes in Norrie disease patients with an X-chromosomal deletion affecting monoamine oxidase. J Neurochem. 1990 Jan;54(1):242–247. doi: 10.1111/j.1471-4159.1990.tb13307.x. [DOI] [PubMed] [Google Scholar]
- Newman P. J., Gorski J., White G. C., 2nd, Gidwitz S., Cretney C. J., Aster R. H. Enzymatic amplification of platelet-specific messenger RNA using the polymerase chain reaction. J Clin Invest. 1988 Aug;82(2):739–743. doi: 10.1172/JCI113656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozelius L., Gusella J. F., Breakefield X. O. MspI RFLP for human MAOA gene. Nucleic Acids Res. 1989 Dec 25;17(24):10516–10516. doi: 10.1093/nar/17.24.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozelius L., Hsu Y. P., Bruns G., Powell J. F., Chen S., Weyler W., Utterback M., Zucker D., Haines J., Trofatter J. A. Human monoamine oxidase gene (MAOA): chromosome position (Xp21-p11) and DNA polymorphism. Genomics. 1988 Jul;3(1):53–58. doi: 10.1016/0888-7543(88)90159-0. [DOI] [PubMed] [Google Scholar]
- Powell J. F., Hsu Y. P., Weyler W., Chen S. A., Salach J., Andrikopoulos K., Mallet J., Breakefield X. O. The primary structure of bovine monoamine oxidase type A. Comparison with peptide sequences of bovine monoamine oxidase type B and other flavoenzymes. Biochem J. 1989 Apr 15;259(2):407–413. doi: 10.1042/bj2590407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice J., McGuffin P., Goldin L. R., Shaskan E. G., Gershon E. S. Platelet monoamine oxidase (MAO) activity: evidence for a single major locus. Am J Hum Genet. 1984 Jan;36(1):36–43. [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims K. B., Ozelius L., Corey T., Rinehart W. B., Liberfarb R., Haines J., Chen W. J., Norio R., Sankila E., de la Chapelle A. Norrie disease gene is distinct from the monoamine oxidase genes. Am J Hum Genet. 1989 Sep;45(3):424–434. [PMC free article] [PubMed] [Google Scholar]
- Sims K. B., de la Chapelle A., Norio R., Sankila E. M., Hsu Y. P., Rinehart W. B., Corey T. J., Ozelius L., Powell J. F., Bruns G. Monoamine oxidase deficiency in males with an X chromosome deletion. Neuron. 1989 Jan;2(1):1069–1076. doi: 10.1016/0896-6273(89)90231-6. [DOI] [PubMed] [Google Scholar]
- Sullivan J. L., Baenziger J. C., Wagner D. L., Rauscher F. P., Nurnberger J. I., Jr, Holmes J. S. Platelet MAO in subtypes of alcoholism. Biol Psychiatry. 1990 Apr 15;27(8):911–922. doi: 10.1016/0006-3223(90)90473-f. [DOI] [PubMed] [Google Scholar]
- Tabakoff B., Hoffman P. L., Lee J. M., Saito T., Willard B., De Leon-Jones F. Differences in platelet enzyme activity between alcoholics and nonalcoholics. N Engl J Med. 1988 Jan 21;318(3):134–139. doi: 10.1056/NEJM198801213180302. [DOI] [PubMed] [Google Scholar]
- Thorpe L. W., Westlund K. N., Kochersperger L. M., Abell C. W., Denney R. M. Immunocytochemical localization of monoamine oxidases A and B in human peripheral tissues and brain. J Histochem Cytochem. 1987 Jan;35(1):23–32. doi: 10.1177/35.1.3025289. [DOI] [PubMed] [Google Scholar]
- WURTMAN R. J., AXELROD J. A SENSITIVE AND SPECIFIC ASSAY FOR THE ESTIMATION OF MONOAMINE OXIDASE. Biochem Pharmacol. 1963 Dec;12:1439–1441. doi: 10.1016/0006-2952(63)90215-6. [DOI] [PubMed] [Google Scholar]
- Warburg M. Norrie's disease--differential diagnosis and treatment. Acta Ophthalmol (Copenh) 1975 Mar;53(2):217–236. doi: 10.1111/j.1755-3768.1975.tb01156.x. [DOI] [PubMed] [Google Scholar]
- Westlund K. N., Denney R. M., Kochersperger L. M., Rose R. M., Abell C. W. Distinct monoamine oxidase A and B populations in primate brain. Science. 1985 Oct 11;230(4722):181–183. doi: 10.1126/science.3875898. [DOI] [PubMed] [Google Scholar]
- Weyler W., Hsu Y. P., Breakefield X. O. Biochemistry and genetics of monoamine oxidase. Pharmacol Ther. 1990;47(3):391–417. doi: 10.1016/0163-7258(90)90064-9. [DOI] [PubMed] [Google Scholar]
- Weyler W., Salach J. I. Purification and properties of mitochondrial monoamine oxidase type A from human placenta. J Biol Chem. 1985 Oct 25;260(24):13199–13207. [PubMed] [Google Scholar]
- de la Chapelle A., Sankila E. M., Lindlöf M., Aula P., Norio R. Norrie disease caused by a gene deletion allowing carrier detection and prenatal diagnosis. Clin Genet. 1985 Oct;28(4):317–320. doi: 10.1111/j.1399-0004.1985.tb00405.x. [DOI] [PubMed] [Google Scholar]