Abstract
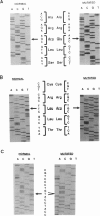
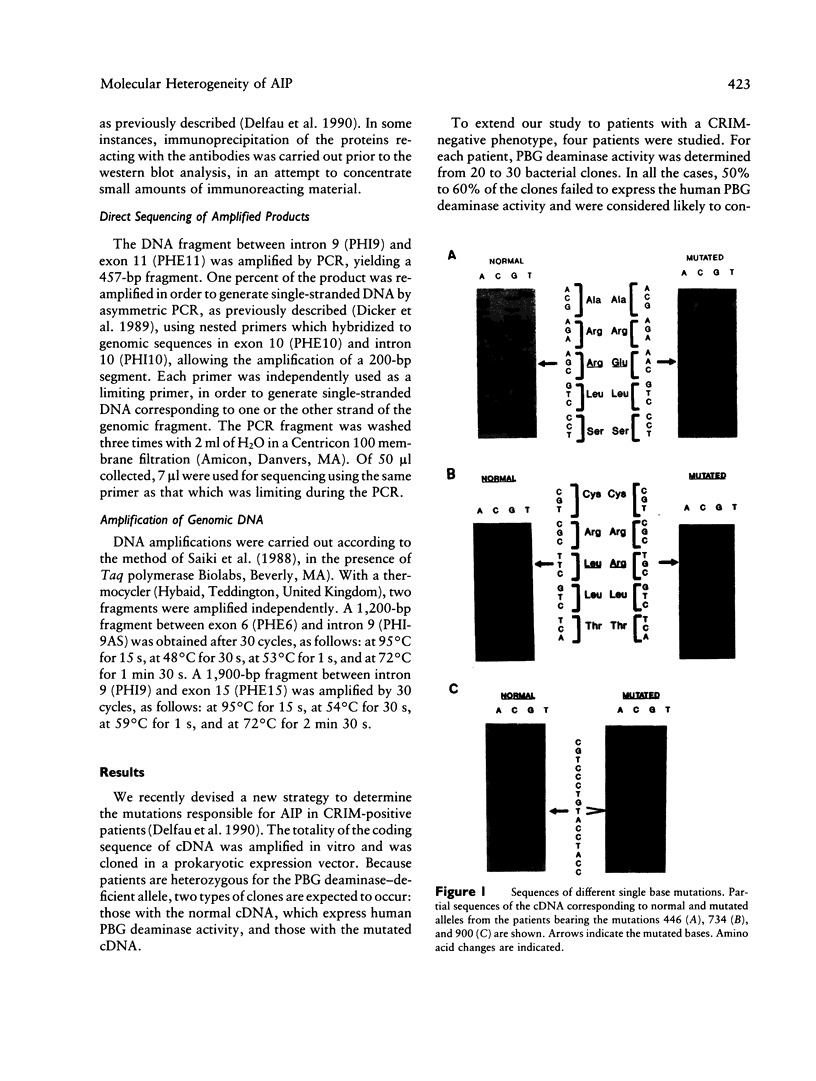
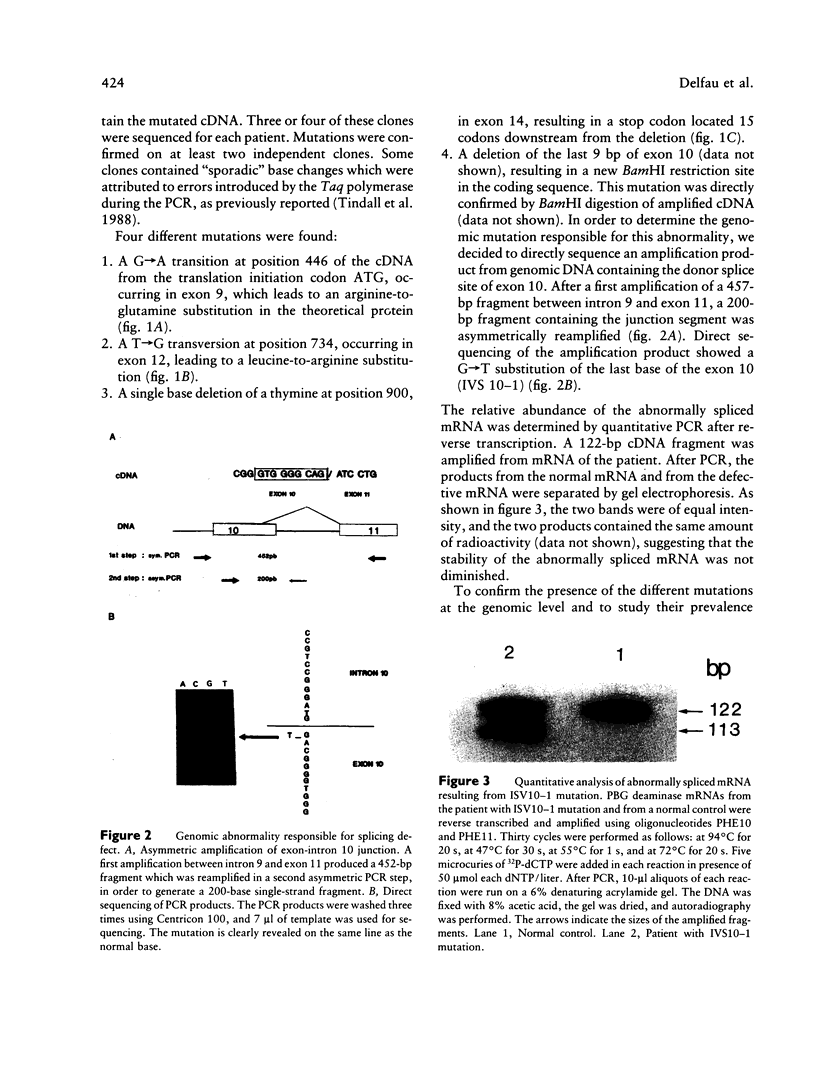
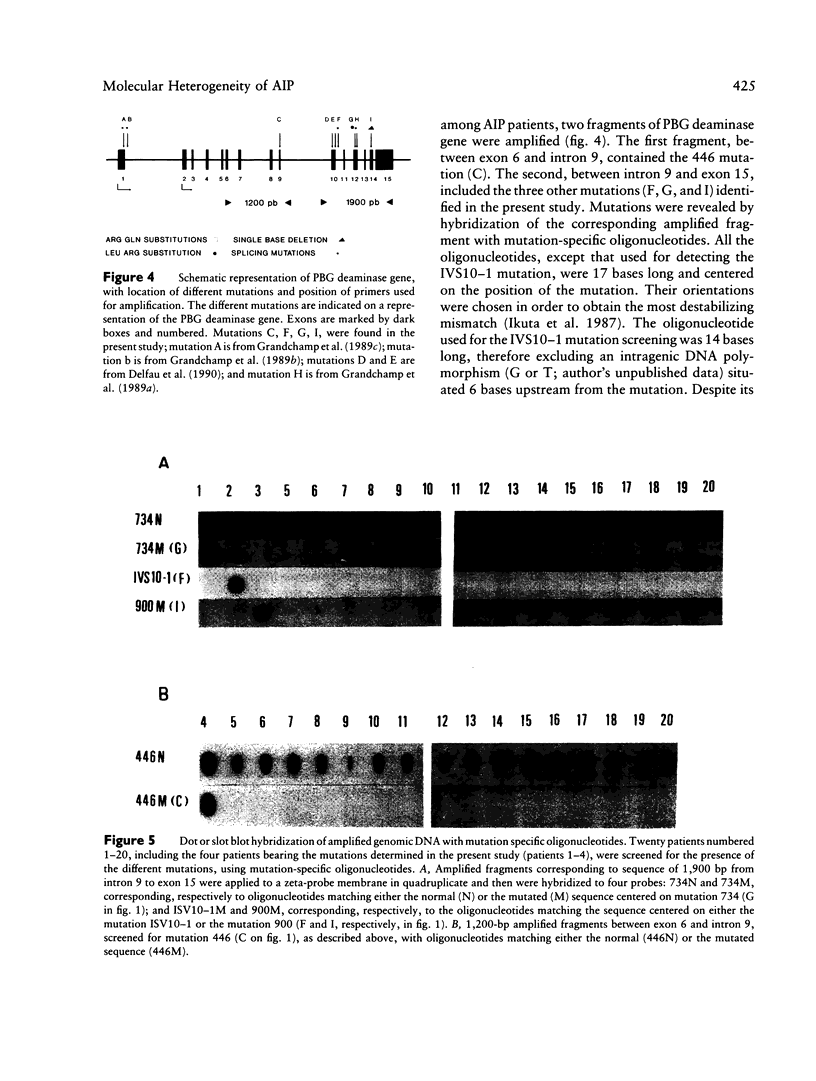
Four mutations of the porphobilinogen (PBG) deaminase gene that result in cross-reacting immunological material (CRIM)-negative forms of acute intermittent porphyria (AIP) have been identified by in vitro amplification of cDNA from patients and by cloning of the amplified products in a bacterial expression vector. One mutation is a single base deletion which causes a frameshift and which is expected to result in the synthesis of a truncated protein. Two other mutations consist of single base substitutions and lead to amino acid changes. The fourth mutation is a single base substitution producing an aberrant splicing and resulting in an mRNA which would encode a protein missing three amino acids. DNAs from 16 unrelated CRIM-negative AIP patients were screened for the presence of these four mutations, by hybridization with oligonucleotides specific for each of the mutations, but none of the four mutations was identified in additional patients. The results indicate that mutations responsible for CRIM-negative AIP are highly heterogenous.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Delfau M. H., Picat C., de Rooij F. W., Hamer K., Bogard M., Wilson J. H., Deybach J. C., Nordmann Y., Grandchamp B. Two different point G to A mutations in exon 10 of the porphobilinogen deaminase gene are responsible for acute intermittent porphyria. J Clin Invest. 1990 Nov;86(5):1511–1516. doi: 10.1172/JCI114869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnick R. J., Ostasiewicz L. T., Tishler P. A., Mustajoki P. Acute intermittent porphyria: characterization of a novel mutation in the structural gene for porphobilinogen deaminase. Demonstration of noncatalytic enzyme intermediates stabilized by bound substrate. J Clin Invest. 1985 Aug;76(2):865–874. doi: 10.1172/JCI112044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker A. P., Volkenandt M., Adamo A., Barreda C., Bertino J. R. Sequence analysis of a human gene responsible for drug resistance: a rapid method for manual and automated direct sequencing of products generated by the polymerase chain reaction. Biotechniques. 1989 Sep;7(8):830–838. [PubMed] [Google Scholar]
- Grandchamp B., Nordmann Y. Enzymes of the heme biosynthesis pathway: recent advances in molecular genetics. Semin Hematol. 1988 Oct;25(4):303–311. [PubMed] [Google Scholar]
- Grandchamp B., Picat C., Kauppinen R., Mignotte V., Peltonen L., Mustajoki P., Roméo P. H., Goossens M., Nordmann Y. Molecular analysis of acute intermittent porphyria in a Finnish family with normal erythrocyte porphobilinogen deaminase. Eur J Clin Invest. 1989 Oct;19(5):415–418. doi: 10.1111/j.1365-2362.1989.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Grandchamp B., Picat C., Mignotte V., Wilson J. H., Te Velde K., Sandkuyl L., Roméo P. H., Goossens M., Nordmann Y. Tissue-specific splicing mutation in acute intermittent porphyria. Proc Natl Acad Sci U S A. 1989 Jan;86(2):661–664. doi: 10.1073/pnas.86.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandchamp B., Picat C., de Rooij F., Beaumont C., Wilson P., Deybach J. C., Nordmann Y. A point mutation G----A in exon 12 of the porphobilinogen deaminase gene results in exon skipping and is responsible for acute intermittent porphyria. Nucleic Acids Res. 1989 Aug 25;17(16):6637–6649. doi: 10.1093/nar/17.16.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta S., Takagi K., Wallace R. B., Itakura K. Dissociation kinetics of 19 base paired oligonucleotide-DNA duplexes containing different single mismatched base pairs. Nucleic Acids Res. 1987 Jan 26;15(2):797–811. doi: 10.1093/nar/15.2.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Anvret M., Lindsten J., Lannfelt L., Gellerfors P., Wetterberg L., Floderus Y., Thunell S. DNA polymorphisms within the porphobilinogen deaminase gene in two Swedish families with acute intermittent porphyria. Hum Genet. 1988 Aug;79(4):379–381. doi: 10.1007/BF00282182. [DOI] [PubMed] [Google Scholar]
- Llewellyn D. H., Elder G. H., Kalsheker N. A., Marsh O. W., Harrison P. R., Grandchamp B., Picat C., Nordmann Y., Romeo P. H., Goossens M. DNA polymorphism of human porphobilinogen deaminase gene in acute intermittent porphyria. Lancet. 1987 Sep 26;2(8561):706–708. doi: 10.1016/s0140-6736(87)91073-7. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Hart G. J., Packman L. C., Battersby A. R. Evidence that the pyrromethane cofactor of hydroxymethylbilane synthase (porphobilinogen deaminase) is bound to the protein through the sulphur atom of cysteine-242. Biochem J. 1988 Sep 15;254(3):915–918. doi: 10.1042/bj2540915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Scobie G. A., Llewellyn D. H., Urquhart A. J., Smyth S. J., Kalsheker N. A., Harrison P. R., Elder G. H. Acute intermittent porphyria caused by a C----T mutation that produces a stop codon in the porphobilinogen deaminase gene. Hum Genet. 1990 Oct;85(6):631–634. doi: 10.1007/BF00193588. [DOI] [PubMed] [Google Scholar]
- Tindall K. R., Kunkel T. A. Fidelity of DNA synthesis by the Thermus aquaticus DNA polymerase. Biochemistry. 1988 Aug 9;27(16):6008–6013. doi: 10.1021/bi00416a027. [DOI] [PubMed] [Google Scholar]
- Umanoff H., Russell C. S., Cosloy S. D. Availability of porphobilinogen controls appearance of porphobilinogen deaminase activity in Escherichia coli K-12. J Bacteriol. 1988 Oct;170(10):4969–4971. doi: 10.1128/jb.170.10.4969-4971.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssoufian H., Antonarakis S. E., Bell W., Griffin A. M., Kazazian H. H., Jr Nonsense and missense mutations in hemophilia A: estimate of the relative mutation rate at CG dinucleotides. Am J Hum Genet. 1988 May;42(5):718–725. [PMC free article] [PubMed] [Google Scholar]
- de Verneuil H., Grandchamp B., Beaumont C., Picat C., Nordmann Y. Uroporphyrinogen decarboxylase structural mutant (Gly281----Glu) in a case of porphyria. Science. 1986 Nov 7;234(4777):732–734. doi: 10.1126/science.3775362. [DOI] [PubMed] [Google Scholar]