Abstract
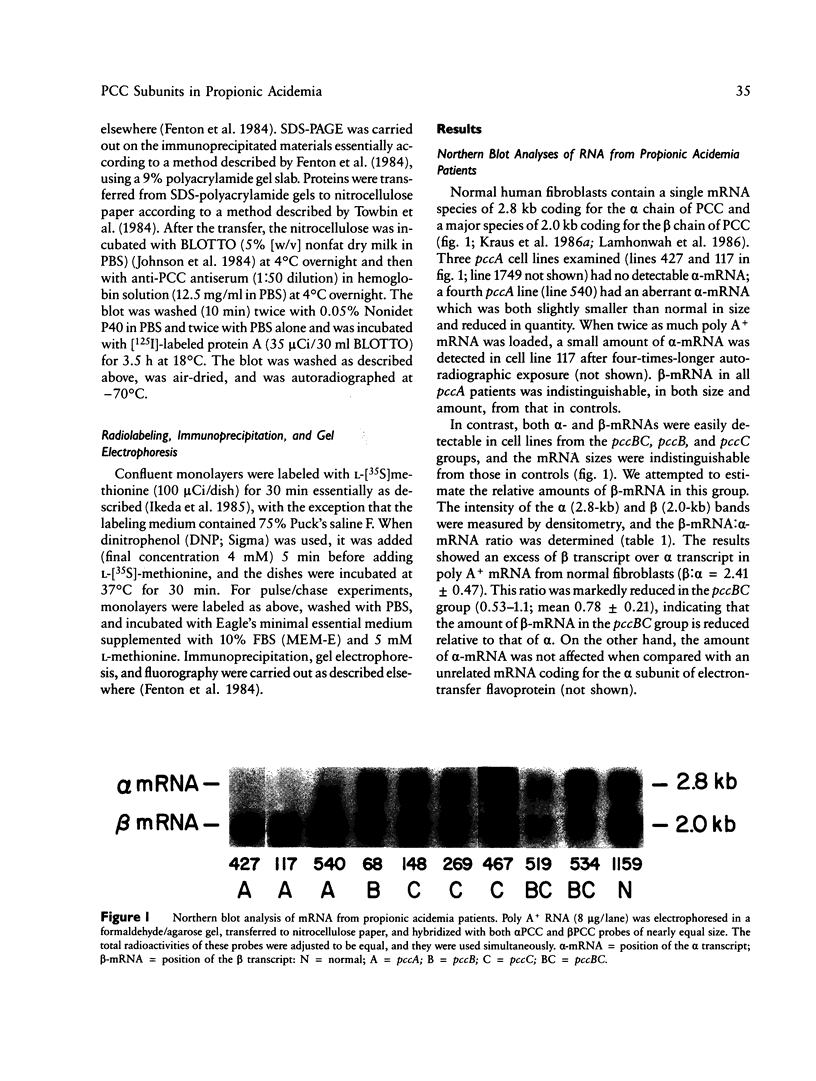
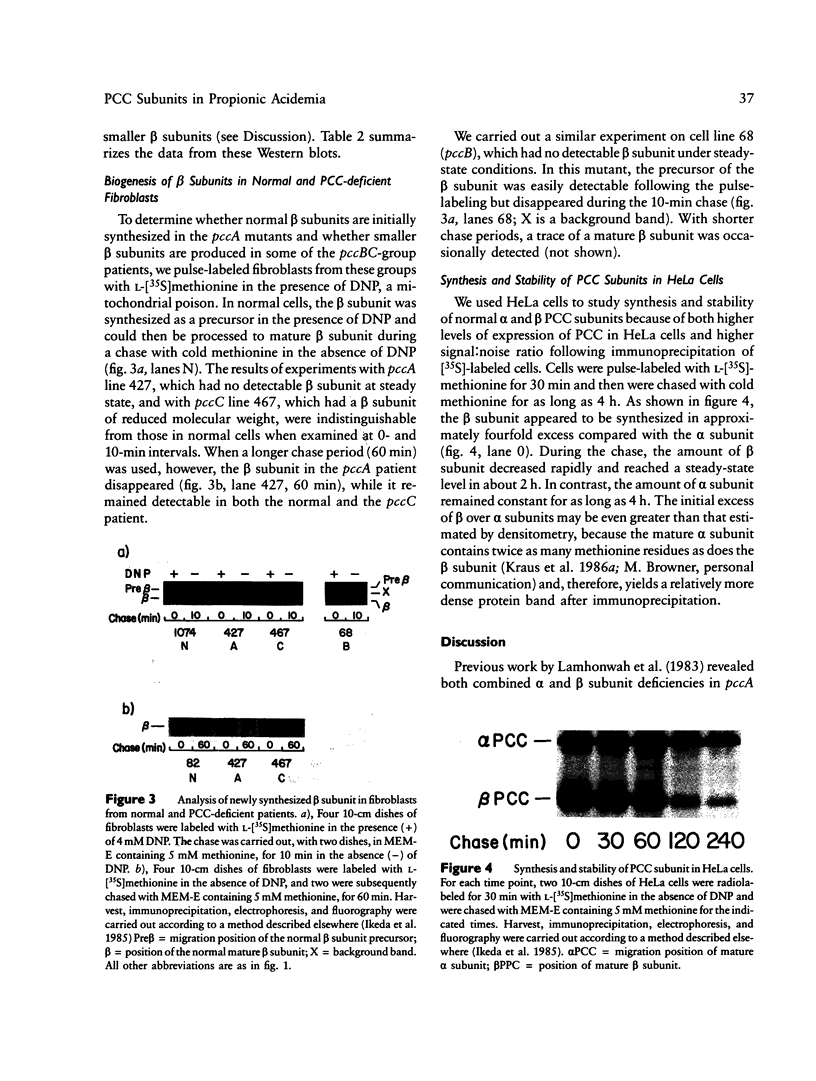
We have characterized further the molecular basis of human inherited propionyl CoA carboxylase deficiency by measuring steady state levels of the mRNAs coding for the enzyme's two protein subunits (alpha and beta) and by estimating initial synthesis and steady state levels of the protein subunits in skin fibroblasts from controls and affected patients. We studied cell lines from both major complementation groups (pccA and pccBC) corresponding, respectively, to defects in the carboxylase's alpha and beta subunits. Analysis of pccA lines revealed the absence of alpha chain mRNA in three and an abnormally small alpha-mRNA in a fourth. Despite the presence of normal beta-mRNA in each of these pccA lines, there was complete absence of both alpha and beta protein subunits under steady state conditions, even though new synthesis and mitochondrial import of beta precursors was normal. Results in nine pccBC lines revealed normal alpha mRNA in each, while the amounts of beta-mRNA were distinctly reduced in every case. Correspondingly, alpha protein subunits were present in normal amounts at steady-state, but beta subunits were uniformly decreased. In addition, in six of the nine beta deficient cell lines, partially degraded beta-subunits were observed. To help interpret these results, synthesis and stability of carboxylase subunits were studied in intact HeLa cells using a pulse-chase protocol. Whereas alpha chains were stable over the four hour interval studied, beta chains--initially synthesized in large excess over alpha chains--were degraded rapidly reaching equivalence with alpha chains after two hours.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fenton W. A., Hack A. M., Helfgott D., Rosenberg L. E. Biogenesis of the mitochondrial enzyme methylmalonyl-CoA mutase. Synthesis and processing of a precursor in a cell-free system and in cultured cells. J Biol Chem. 1984 May 25;259(10):6616–6621. [PubMed] [Google Scholar]
- Fenton W. A., Hack A. M., Kraus J. P., Rosenberg L. E. Immunochemical studies of fibroblasts from patients with methylmalonyl-CoA mutase apoenzyme deficiency: detection of a mutation interfering with mitochondrial import. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1421–1424. doi: 10.1073/pnas.84.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finocchiaro G., Ito M., Ikeda Y., Tanaka K. Molecular cloning and nucleotide sequence of cDNAs encoding the alpha-subunit of human electron transfer flavoprotein. J Biol Chem. 1988 Oct 25;263(30):15773–15780. [PubMed] [Google Scholar]
- Geever R. F., Wilson L. B., Nallaseth F. S., Milner P. F., Bittner M., Wilson J. T. Direct identification of sickle cell anemia by blot hybridization. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5081–5085. doi: 10.1073/pnas.78.8.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravel R. A., Lam K. F., Scully K. J., Hsia Y. Genetic complementation of propionyl-CoA carboxylase deficiency in cultured human fibroblasts. Am J Hum Genet. 1977 Jul;29(4):378–388. [PMC free article] [PubMed] [Google Scholar]
- Haase F. C., Beegen H., Allen S. H. Propionyl-coenzyme A carboxylase of Mycobacterium smegmatis. An electron microscopic study. Eur J Biochem. 1984 Apr 2;140(1):147–151. doi: 10.1111/j.1432-1033.1984.tb08078.x. [DOI] [PubMed] [Google Scholar]
- Hsia Y. E., Scully K. J., Rosenberg L. E. Inherited propionyl-Coa carboxylase deficiency in "ketotic hyperglycinemia". J Clin Invest. 1971 Jan;50(1):127–130. doi: 10.1172/JCI106466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Keese S. M., Tanaka K. Molecular heterogeneity of variant isovaleryl-CoA dehydrogenase from cultured isovaleric acidemia fibroblasts. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7081–7085. doi: 10.1073/pnas.82.20.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalousek F., Darigo M. D., Rosenberg L. E. Isolation and characterization of propionyl-CoA carboxylase from normal human liver. Evidence for a protomeric tetramer of nonidentical subunits. J Biol Chem. 1980 Jan 10;255(1):60–65. [PubMed] [Google Scholar]
- Kraus J. P., Firgaira F., Novotný J., Kalousek F., Williams K. R., Williamson C., Ohura T., Rosenberg L. E. Coding sequence of the precursor of the beta subunit of rat propionyl-CoA carboxylase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8049–8053. doi: 10.1073/pnas.83.21.8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus J. P., Kalousek F., Rosenberg L. E. Biosynthesis and mitochondrial processing of the beta subunit of propionyl coenzyme A carboxylase from rat liver. J Biol Chem. 1983 Jun 25;258(12):7245–7248. [PubMed] [Google Scholar]
- Kraus J. P., Williamson C. L., Firgaira F. A., Yang-Feng T. L., Münke M., Francke U., Rosenberg L. E. Cloning and screening with nanogram amounts of immunopurified mRNAs: cDNA cloning and chromosomal mapping of cystathionine beta-synthase and the beta subunit of propionyl-CoA carboxylase. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2047–2051. doi: 10.1073/pnas.83.7.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam Hon Wah A. M., Lam K. F., Tsui F., Robinson B., Saunders M. E., Gravel R. A. Assignment of the alpha and beta chains of human propionyl-CoA carboxylase to genetic complementation groups. Am J Hum Genet. 1983 Sep;35(5):889–899. [PMC free article] [PubMed] [Google Scholar]
- Lamhonwah A. M., Barankiewicz T. J., Willard H. F., Mahuran D. J., Quan F., Gravel R. A. Isolation of cDNA clones coding for the alpha and beta chains of human propionyl-CoA carboxylase: chromosomal assignments and DNA polymorphisms associated with PCCA and PCCB genes. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4864–4868. doi: 10.1073/pnas.83.13.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamhonwah A. M., Gravel R. A. Propionicacidemia: absence of alpha-chain mRNA in fibroblasts from patients of the pccA complementation group. Am J Hum Genet. 1987 Dec;41(6):1124–1131. [PMC free article] [PubMed] [Google Scholar]
- Lehnert M. E., Lodish H. F. Unequal synthesis and differential degradation of alpha and beta spectrin during murine erythroid differentiation. J Cell Biol. 1988 Aug;107(2):413–426. doi: 10.1083/jcb.107.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen R., Fox J., Fenton W. A., Horwich A. L., Rosenberg L. E. Gene deletion and restriction fragment length polymorphisms at the human ornithine transcarbamylase locus. 1985 Feb 28-Mar 6Nature. 313(6005):815–817. doi: 10.1038/313815a0. [DOI] [PubMed] [Google Scholar]
- Saunders M., Sweetman L., Robinson B., Roth K., Cohn R., Gravel R. A. Biotin-response organicaciduria. Multiple carboxylase defects and complementation studies with propionicacidemia in cultured fibroblasts. J Clin Invest. 1979 Dec;64(6):1695–1702. doi: 10.1172/JCI109632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovby F., Kraus J. P., Rosenberg L. E. Homocystinuria: biogenesis of cystathionine beta-synthase subunits in cultured fibroblasts and in an in vitro translation system programmed with fibroblast messenger RNA. Am J Hum Genet. 1984 Mar;36(2):452–459. [PMC free article] [PubMed] [Google Scholar]
- Skovby F., Kraus J., Redlich C., Rosenberg L. E. Immunochemical studies on cultured fibroblasts from patients with homocystinuria due to cystathionine beta-synthase deficiency. Am J Hum Genet. 1982 Jan;34(1):73–83. [PMC free article] [PubMed] [Google Scholar]
- Wexler I. D., Kerr D. S., Ho L., Lusk M. M., Pepin R. A., Javed A. A., Mole J. E., Jesse B. W., Thekkumkara T. J., Pons G. Heterogeneous expression of protein and mRNA in pyruvate dehydrogenase deficiency. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7336–7340. doi: 10.1073/pnas.85.19.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf B., Rosenberg L. E. Heterozygote expression in propionyl coenzyme A carboxylase deficiency. Differences between major complementation groups. J Clin Invest. 1978 Nov;62(5):931–936. doi: 10.1172/JCI109221. [DOI] [PMC free article] [PubMed] [Google Scholar]