Abstract
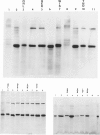
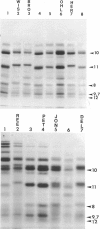
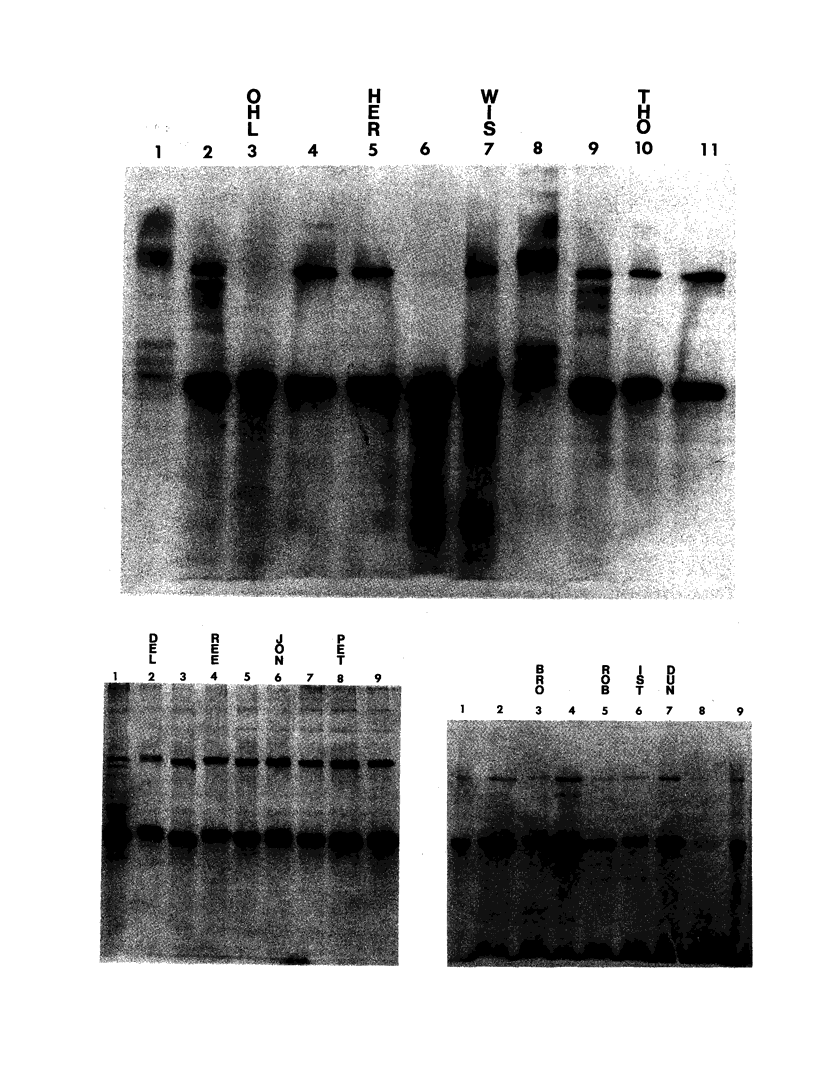
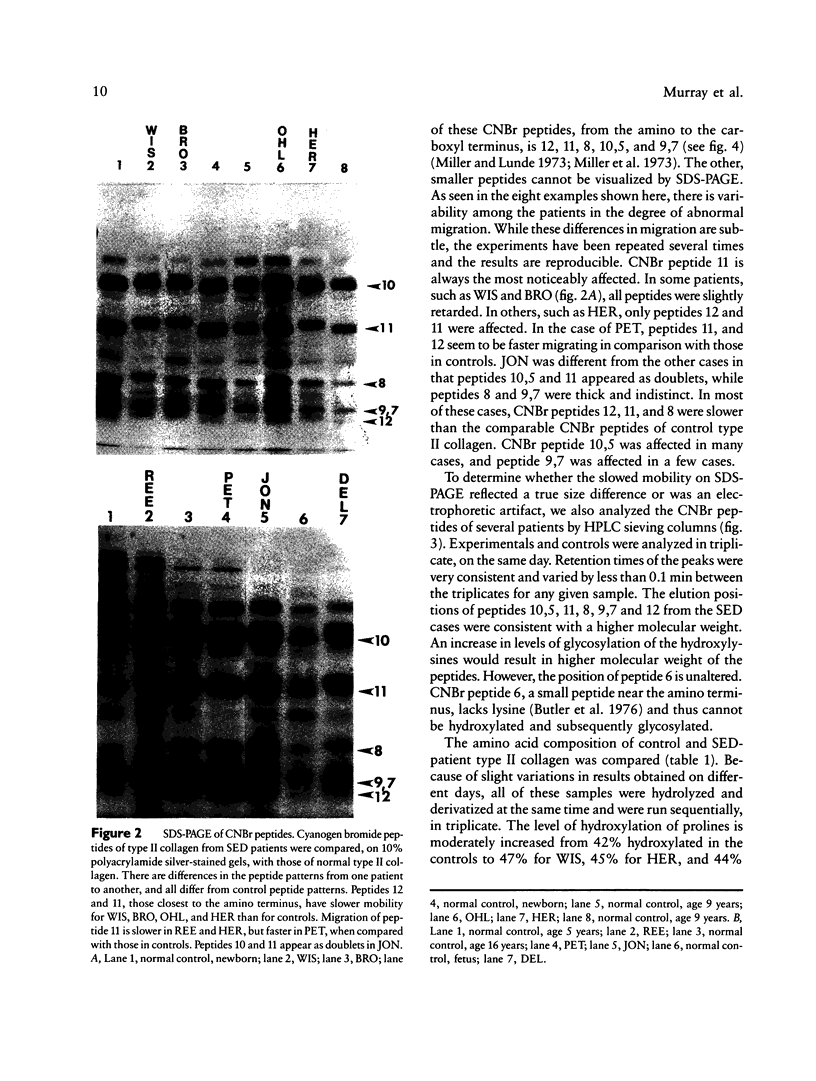
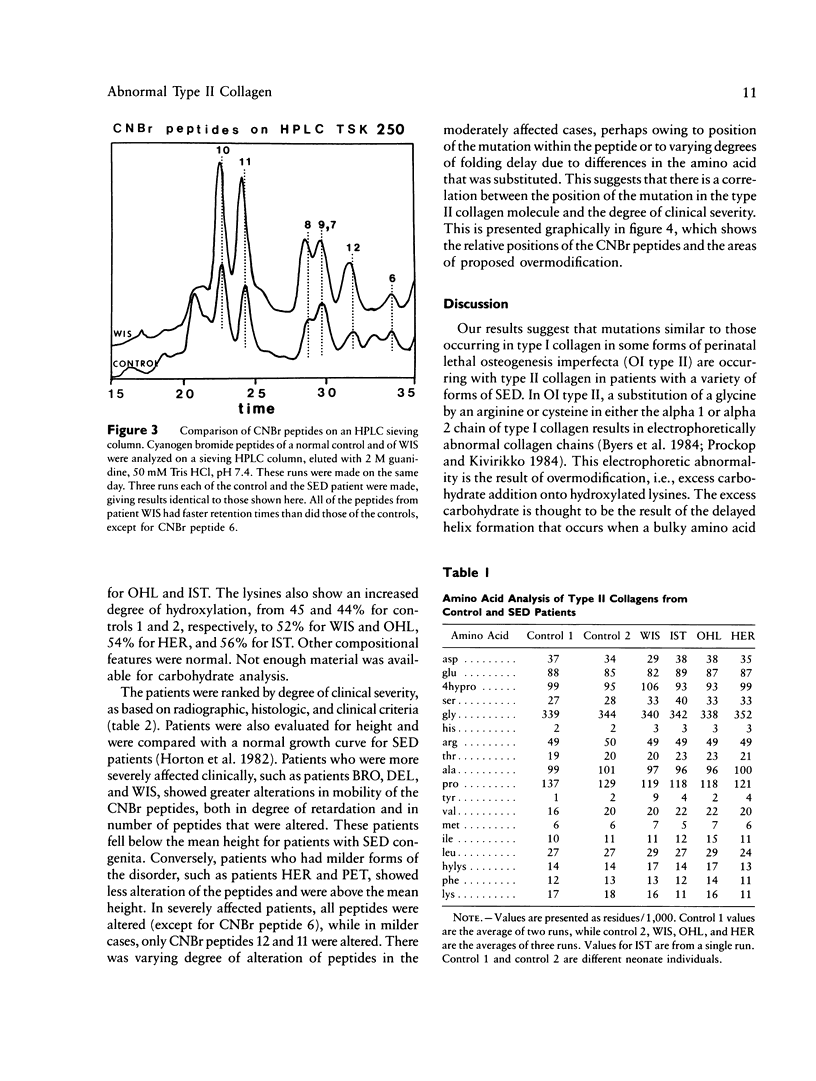
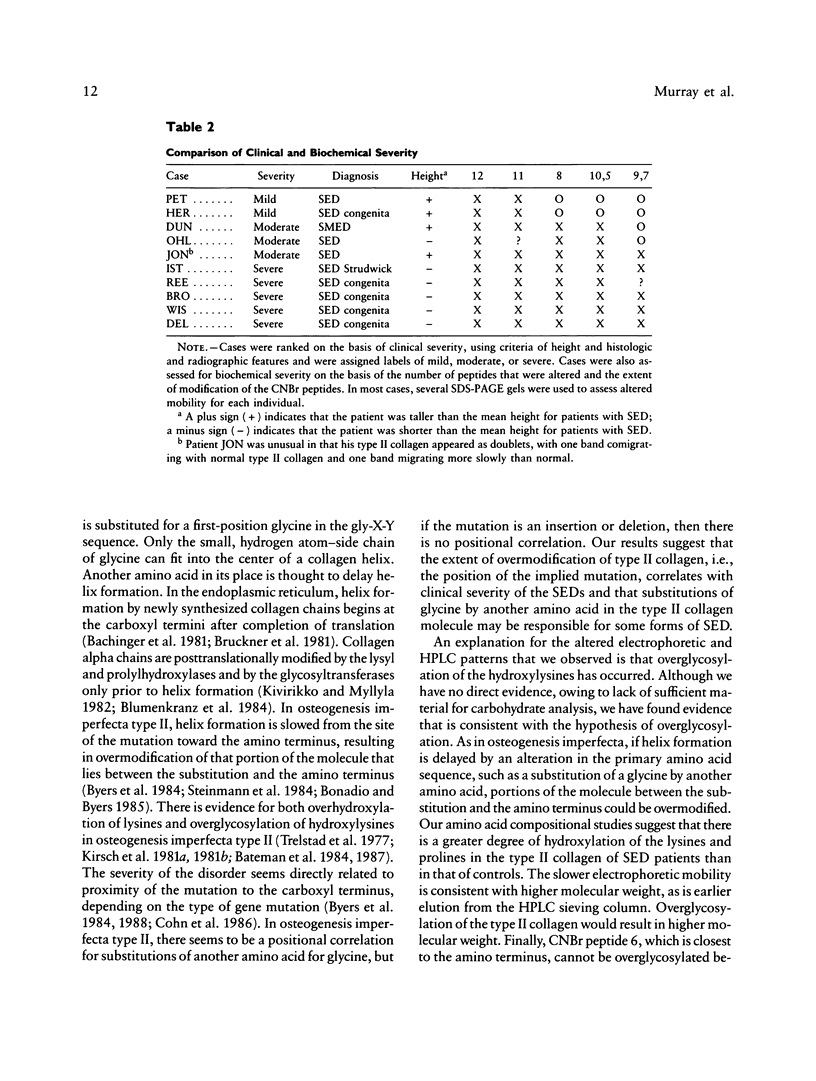
The spondyloepiphyseal dysplasias (SEDs) and spondyloepimetaphyseal dysplasias (SEMDs) are a heterogeneous group of skeletal dysplasias (dwarfing disorders) characterized by abnormal epiphyses, with and without varying degrees of metaphyseal irregularities, flattened vertebral bodies, and myopia. To better define the underlying cause of these disorders, we have analyzed the collagens from costal cartilage from several of these patients, using SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and high-performance liquid chromatography (HPLC) of intact chains and cyanogen bromide (CNBr) peptides and amino acid analysis. In almost all of the patients in this study group, the type II collagen exhibited a slower electrophoretic mobility when compared with that in normal controls. The mobility of many, but not all, of the CNBr peptides was also retarded. Peptides near the amino terminus were almost always altered, while the mobility of peptides close to the carboxyl terminus were normal in all but the severely affected cases. Analysis of the CNBr peptides on an HPLC sieving column confirmed that the electrophoretically abnormal peptides were of a higher molecular weight than were control peptides. Amino acid analysis indicated that the abnormal collagens have a higher ratio of hydroxylysine to lysine than does control collagen, suggesting that overmodification may be involved in the altered mobility. Our results are consistent with a defect in the collagen helix that results in overmodification of the molecule from that point toward the amino terminus. We propose that some forms of SED and SEMD are associated with abnormalities in type II collagen that results in delayed helix formation and consequent overmodification of the collagen. Cases of SED fit onto a continuous spectrum of clinical severity that correlates positively with both the extent of alteration and the proximity of the defect to the carboxyl terminus.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. E., Sillence D. O., Lachman R. S., Toomey K., Bull M., Dorst J., Rimoin D. L. Spondylometepiphyseal dysplasia, Strudwick type. Am J Med Genet. 1982 Nov;13(3):243–256. doi: 10.1002/ajmg.1320130304. [DOI] [PubMed] [Google Scholar]
- Bateman J. F., Chan D., Walker I. D., Rogers J. G., Cole W. G. Lethal perinatal osteogenesis imperfecta due to the substitution of arginine for glycine at residue 391 of the alpha 1(I) chain of type I collagen. J Biol Chem. 1987 May 25;262(15):7021–7027. [PubMed] [Google Scholar]
- Bateman J. F., Mascara T., Chan D., Cole W. G. Abnormal type I collagen metabolism by cultured fibroblasts in lethal perinatal osteogenesis imperfecta. Biochem J. 1984 Jan 1;217(1):103–115. doi: 10.1042/bj2170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz H., Morris N. P., Murray L. W., Sakai L. Y., Hollister D. W., Burgeson R. E. Isolation and partial characterization of a new human collagen with an extended triple-helical structural domain. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3168–3172. doi: 10.1073/pnas.80.11.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz N., Assad R., Peterkofsky B. Characterization of collagen hydroxylysyl glycosyltransferases as mainly intramembranous microsomal enzymes. J Biol Chem. 1984 Jan 25;259(2):854–859. [PubMed] [Google Scholar]
- Bonadio J., Byers P. H. Subtle structural alterations in the chains of type I procollagen produce osteogenesis imperfecta type II. Nature. 1985 Jul 25;316(6026):363–366. doi: 10.1038/316363a0. [DOI] [PubMed] [Google Scholar]
- Bruckner P., Eikenberry E. F., Prockop D. J. Formation of the triple helix of type I procollagen in cellulo. A kinetic model based on cis-trans isomerization of peptide bonds. Eur J Biochem. 1981 Sep 1;118(3):607–613. doi: 10.1111/j.1432-1033.1981.tb05562.x. [DOI] [PubMed] [Google Scholar]
- Burgeson R. E., Hollister D. W. Collagen heterogeneity in human cartilage: identification of several new collagen chains. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1124–1131. doi: 10.1016/s0006-291x(79)80024-8. [DOI] [PubMed] [Google Scholar]
- Butler W. T., Miller E. J., Finch J. E., Jr The covalent structure of cartilage collagen. Amino acid sequence of the NH2-terminal helical portion of the alpha 1 (II) chain. Biochemistry. 1976 Jul 13;15(14):3000–3006. doi: 10.1021/bi00659a010. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Bonadio J. F., Steinmann B. Osteogenesis imperfecta: update and perspective. Am J Med Genet. 1984 Feb;17(2):429–435. doi: 10.1002/ajmg.1320170206. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Tsipouras P., Bonadio J. F., Starman B. J., Schwartz R. C. Perinatal lethal osteogenesis imperfecta (OI type II): a biochemically heterogeneous disorder usually due to new mutations in the genes for type I collagen. Am J Hum Genet. 1988 Feb;42(2):237–248. [PMC free article] [PubMed] [Google Scholar]
- Bächinger H. P., Fessler L. I., Timpl R., Fessler J. H. Chain assembly intermediate in the biosynthesis of type III procollagen in chick embryo blood vessels. J Biol Chem. 1981 Dec 25;256(24):13193–13199. [PubMed] [Google Scholar]
- Cohn D. H., Byers P. H., Steinmann B., Gelinas R. E. Lethal osteogenesis imperfecta resulting from a single nucleotide change in one human pro alpha 1(I) collagen allele. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6045–6047. doi: 10.1073/pnas.83.16.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton W. A., Hall J. G., Scott C. I., Pyeritz R. E., Rimoin D. L. Growth curves for height for diastrophic dysplasia, spondyloepiphyseal dysplasia congenita, and pseudoachondroplasia. Am J Dis Child. 1982 Apr;136(4):316–319. doi: 10.1001/archpedi.1982.03970400034010. [DOI] [PubMed] [Google Scholar]
- Kirsch E., Glanville R. W., Krieg T., Müller P. Analysis of cyanogen bromide peptides of type I collagen from a patient with lethal osteogenesis imperfecta. Biochem J. 1983 Jun 1;211(3):599–603. doi: 10.1042/bj2110599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch E., Krieg T., Remberger K., Fendel H., Bruckner P., Müller P. K. Disorder of collagen metabolism in a patient with osteogenesis imperfecta (lethal type): increased degree of hydroxylation of lysine in collagen types I and III. Eur J Clin Invest. 1981 Feb;11(1):39–47. doi: 10.1111/j.1365-2362.1981.tb01763.x. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Posttranslational enzymes in the biosynthesis of collagen: intracellular enzymes. Methods Enzymol. 1982;82(Pt A):245–304. doi: 10.1016/0076-6879(82)82067-3. [DOI] [PubMed] [Google Scholar]
- Lachman R. S., Rimoin D. L., Hall J. G., Kozlowski K., Langer L. O., Jr, Scott C. I., Jr, Spranger J. Difficulties in the classification of the epiphyseal dysplasias. Birth Defects Orig Artic Ser. 1975;11(6):231–248. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Lunde L. G. Isolation and characterization of the cyanogen bromide peptides from the alpha 1(II) chain of bovine and human cartilage collagen. Biochemistry. 1973 Aug 14;12(17):3153–3159. doi: 10.1021/bi00741a003. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Rhodes R. K., Furuto D. K. Identification of collagen chains as a function of cyanogen bromide peptide patterns using gel permeation high-performance liquid chromatography. Coll Relat Res. 1983 Mar;3(2):79–87. doi: 10.1016/s0174-173x(83)80035-1. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Woodall D. L., Vail M. S. Biosynthesis of cartilage collagen. Use of pulse labeling to order the cyanogen bromide peptides in the alpha L(II) chain. J Biol Chem. 1973 Mar 10;248(5):1666–1671. [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Heritable diseases of collagen. N Engl J Med. 1984 Aug 9;311(6):376–386. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- Sillence D. O., Horton W. A., Rimoin D. L. Morphologic studies in the skeletal dysplasias. Am J Pathol. 1979 Sep;96(3):813–870. [PMC free article] [PubMed] [Google Scholar]
- Spranger J. W., Langer L. O., Jr Spondyloepiphyseal dysplasia congenita. Radiology. 1970 Feb;94(2):313–322. doi: 10.1148/94.2.313. [DOI] [PubMed] [Google Scholar]
- Spranger J. Spondyloepiphyseal dysplasias. Birth Defects Orig Artic Ser. 1975;11(6):177–182. [PubMed] [Google Scholar]
- Steinmann B., Rao V. H., Vogel A., Bruckner P., Gitzelmann R., Byers P. H. Cysteine in the triple-helical domain of one allelic product of the alpha 1(I) gene of type I collagen produces a lethal form of osteogenesis imperfecta. J Biol Chem. 1984 Sep 10;259(17):11129–11138. [PubMed] [Google Scholar]
- Traub W., Steinmann B. Structural study of a mutant type I collagen from a patient with lethal osteogenesis imperfecta containing an intramolecular disulfide bond in the triple-helical domain. FEBS Lett. 1986 Mar 31;198(2):213–216. doi: 10.1016/0014-5793(86)80407-0. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Rubin D., Gross J. Osteogenesis imperfecta congenita: evidence for a generalized molecular disorder of collagen. Lab Invest. 1977 May;36(5):501–508. [PubMed] [Google Scholar]