Abstract
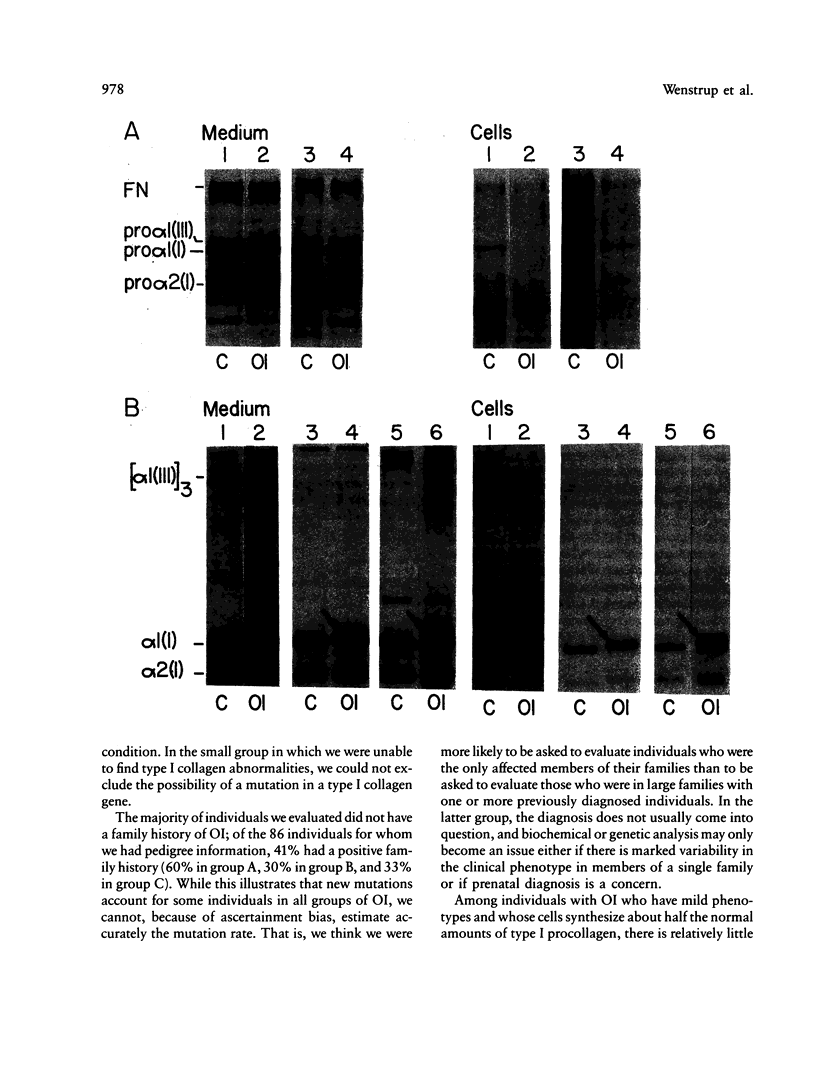
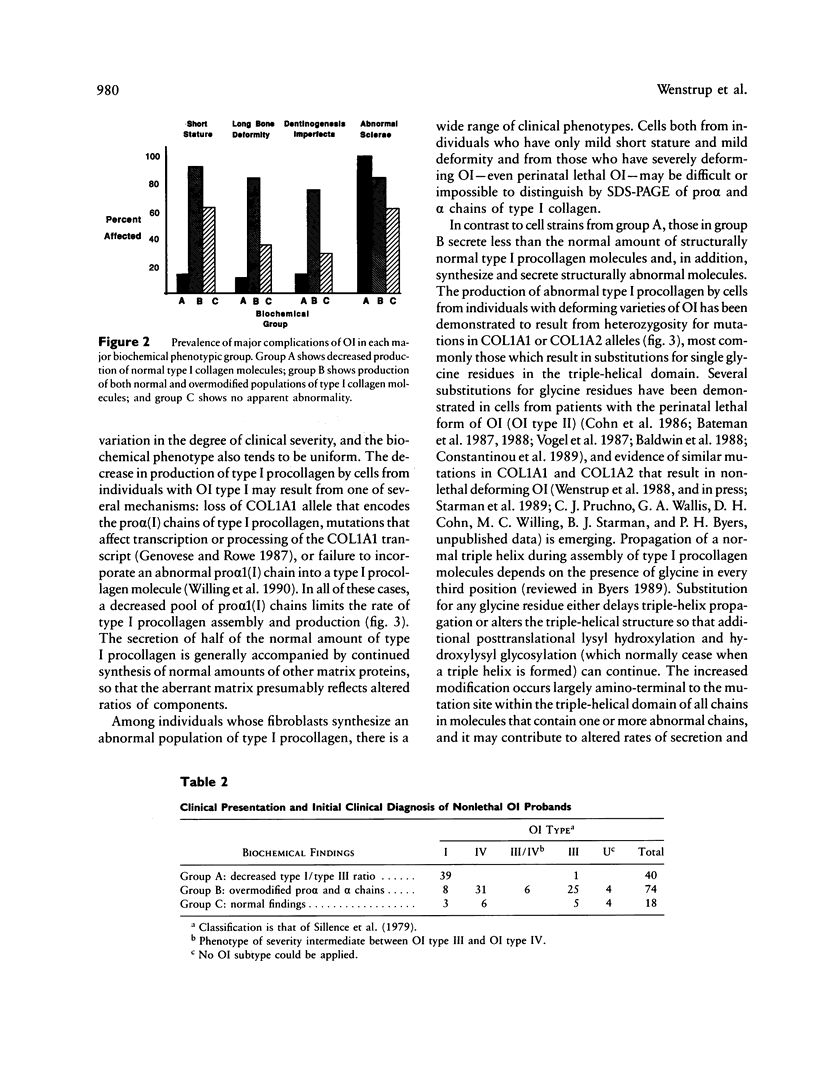
We reviewed clinical and biochemical findings from 132 probands with nonlethal forms of osteogenesis imperfecta (OI) whose fibroblasts were sent to the University of Washington for diagnostic studies in the years 1981-87. In cells from 86% of probands with nonlethal OI we identified biochemical alterations compatible with heterozygosity for a mutation that affected expression or structure of alpha chains of type I procollagen. We observed two major biochemical phenotypes. Cells from 40 probands (group A) secreted about half the normal amount of normal type I procollagen and no identifiable abnormal molecules; these patients were generally of normal stature, rarely had bone deformity or dentinogenesis imperfecta, and had blue sclerae. Cells from 74 probands (group B) produced and secreted normal and abnormal type I procollagen molecules; these patients were usually short and had bone deformity and dentinogenesis imperfecta, and many had grey or blue-grey sclerae. In cells from an additional 18 probands (group C) we were unable to identify altered type I procollagen synthesis or structure. Detection of these abnormalities has value in the determination of mode of inheritance and in the prediction of clinical severity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin C. T., Constantinou C. D., Dumars K. W., Prockop D. J. A single base mutation that converts glycine 907 of the alpha 2(I) chain of type I procollagen to aspartate in a lethal variant of osteogenesis imperfecta. The single amino acid substitution near the carboxyl terminus destabilizes the whole triple helix. J Biol Chem. 1989 Feb 15;264(5):3002–3006. [PubMed] [Google Scholar]
- Barsh G. S., David K. E., Byers P. H. Type I osteogenesis imperfecta: a nonfunctional allele for pro alpha 1 (I) chains of type I procollagen. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3838–3842. doi: 10.1073/pnas.79.12.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Chan D., Walker I. D., Rogers J. G., Cole W. G. Lethal perinatal osteogenesis imperfecta due to the substitution of arginine for glycine at residue 391 of the alpha 1(I) chain of type I collagen. J Biol Chem. 1987 May 25;262(15):7021–7027. [PubMed] [Google Scholar]
- Bateman J. F., Lamande S. R., Dahl H. H., Chan D., Cole W. G. Substitution of arginine for glycine 664 in the collagen alpha 1(I) chain in lethal perinatal osteogenesis imperfecta. Demonstration of the peptide defect by in vitro expression of the mutant cDNA. J Biol Chem. 1988 Aug 25;263(24):11627–11630. [PubMed] [Google Scholar]
- Bonadio J., Byers P. H. Subtle structural alterations in the chains of type I procollagen produce osteogenesis imperfecta type II. Nature. 1985 Jul 25;316(6026):363–366. doi: 10.1038/316363a0. [DOI] [PubMed] [Google Scholar]
- Bonadio J., Holbrook K. A., Gelinas R. E., Jacob J., Byers P. H. Altered triple helical structure of type I procollagen in lethal perinatal osteogenesis imperfecta. J Biol Chem. 1985 Feb 10;260(3):1734–1742. [PubMed] [Google Scholar]
- Byers P. H., Tsipouras P., Bonadio J. F., Starman B. J., Schwartz R. C. Perinatal lethal osteogenesis imperfecta (OI type II): a biochemically heterogeneous disorder usually due to new mutations in the genes for type I collagen. Am J Hum Genet. 1988 Feb;42(2):237–248. [PMC free article] [PubMed] [Google Scholar]
- Cohn D. H., Byers P. H., Steinmann B., Gelinas R. E. Lethal osteogenesis imperfecta resulting from a single nucleotide change in one human pro alpha 1(I) collagen allele. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6045–6047. doi: 10.1073/pnas.83.16.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou C. D., Nielsen K. B., Prockop D. J. A lethal variant of osteogenesis imperfecta has a single base mutation that substitutes cysteine for glycine 904 of the alpha 1(I) chain of type I procollagen. The asymptomatic mother has an unidentified mutation producing an overmodified and unstable type I procollagen. J Clin Invest. 1989 Feb;83(2):574–584. doi: 10.1172/JCI113920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese C., Rowe D. Analysis of cytoplasmic and nuclear messenger RNA in fibroblasts from patients with type I osteogenesis imperfecta. Methods Enzymol. 1987;145:223–235. doi: 10.1016/0076-6879(87)45012-x. [DOI] [PubMed] [Google Scholar]
- Sillence D. O., Senn A., Danks D. M. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979 Apr;16(2):101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starman B. J., Eyre D., Charbonneau H., Harrylock M., Weis M. A., Weiss L., Graham J. M., Jr, Byers P. H. Osteogenesis imperfecta. The position of substitution for glycine by cysteine in the triple helical domain of the pro alpha 1(I) chains of type I collagen determines the clinical phenotype. J Clin Invest. 1989 Oct;84(4):1206–1214. doi: 10.1172/JCI114286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes B., Ogilvie D., Wordsworth P., Anderson, Jones N. Osteogenesis imperfecta is linked to both type I collagen structural genes. Lancet. 1986 Jul 12;2(8498):69–72. doi: 10.1016/s0140-6736(86)91609-0. [DOI] [PubMed] [Google Scholar]
- Sykes B., Ogilvie D., Wordsworth P., Wallis G., Mathew C., Beighton P., Nicholls A., Pope F. M., Thompson E., Tsipouras P. Consistent linkage of dominantly inherited osteogenesis imperfecta to the type I collagen loci: COL1A1 and COL1A2. Am J Hum Genet. 1990 Feb;46(2):293–307. [PMC free article] [PubMed] [Google Scholar]
- Tsipouras P., Børresen A. L., Dickson L. A., Berg K., Prockop D. J., Ramirez F. Molecular heterogeneity in the mild autosomal dominant forms of osteogenesis imperfecta. Am J Hum Genet. 1984 Nov;36(6):1172–1179. [PMC free article] [PubMed] [Google Scholar]
- Tsipouras P., Myers J. C., Ramirez F., Prockop D. J. Restriction fragment length polymorphism associated with the pro alpha 2(I) gene of human type I procollagen. Application to a family with an autosomal dominant form of osteogenesis imperfecta. J Clin Invest. 1983 Oct;72(4):1262–1267. doi: 10.1172/JCI111082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel B. E., Minor R. R., Freund M., Prockop D. J. A point mutation in a type I procollagen gene converts glycine 748 of the alpha 1 chain to cysteine and destabilizes the triple helix in a lethal variant of osteogenesis imperfecta. J Biol Chem. 1987 Oct 25;262(30):14737–14744. [PubMed] [Google Scholar]
- Wallis G., Beighton P., Boyd C., Mathew C. G. Mutations linked to the pro alpha 2(I) collagen gene are responsible for several cases of osteogenesis imperfecta type I. J Med Genet. 1986 Oct;23(5):411–416. doi: 10.1136/jmg.23.5.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenstrup R. J., Cohn D. H., Cohen T., Byers P. H. Arginine for glycine substitution in the triple-helical domain of the products of one alpha 2(I) collagen allele (COL1A2) produces the osteogenesis imperfecta type IV phenotype. J Biol Chem. 1988 Jun 5;263(16):7734–7740. [PubMed] [Google Scholar]
- Wenstrup R. J., Hunter A. G., Byers P. H. Osteogenesis imperfecta type IV: evidence of abnormal triple helical structure of type I collagen. Hum Genet. 1986 Sep;74(1):47–53. doi: 10.1007/BF00278784. [DOI] [PubMed] [Google Scholar]
- Willing M. C., Cohn D. H., Byers P. H. Frameshift mutation near the 3' end of the COL1A1 gene of type I collagen predicts an elongated Pro alpha 1(I) chain and results in osteogenesis imperfecta type I. J Clin Invest. 1990 Jan;85(1):282–290. doi: 10.1172/JCI114424. [DOI] [PMC free article] [PubMed] [Google Scholar]