Abstract
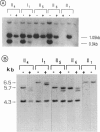
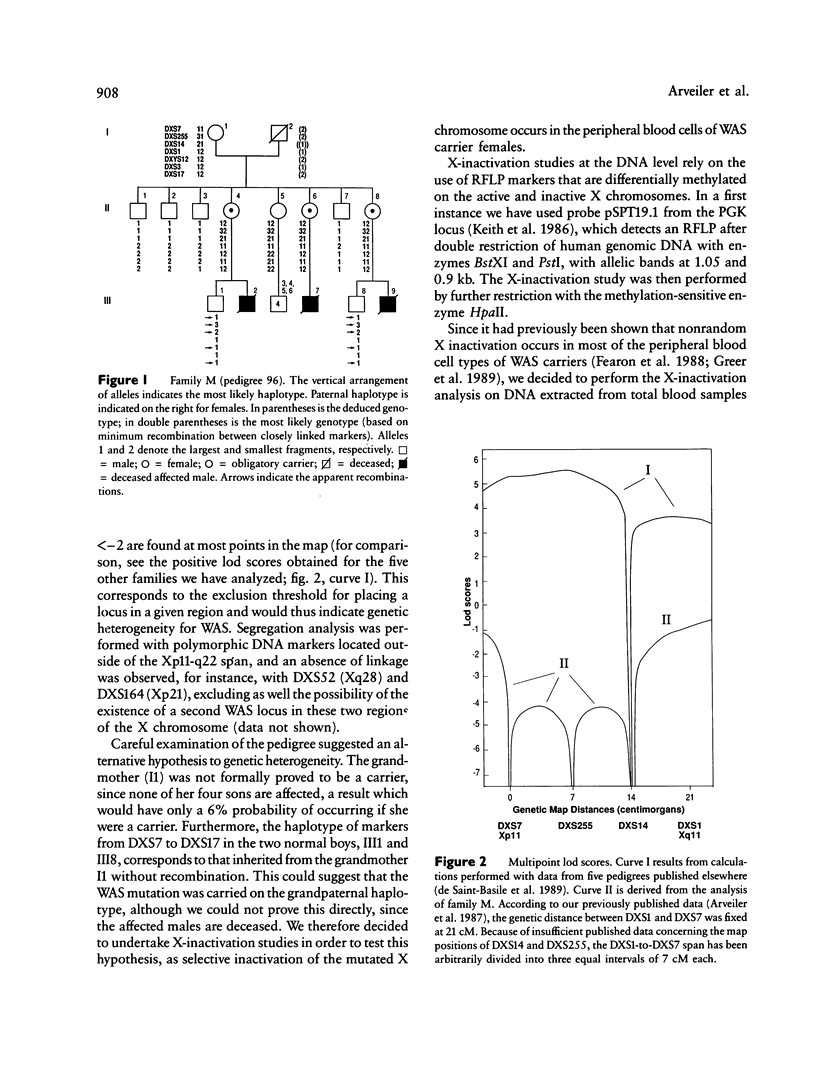
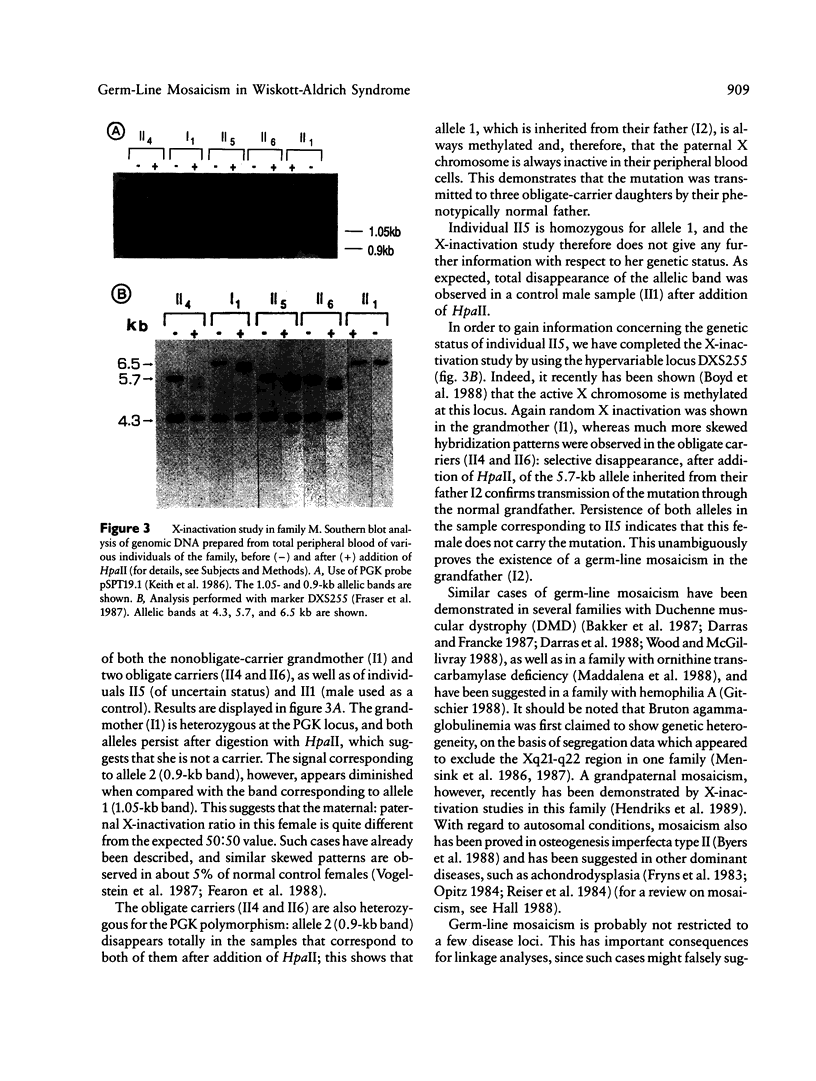
The Wiskott-Aldrich syndrome (IMD2) is an X-linked recessive immunodeficiency. Initial linkage studies mapped the disease locus on the proximal short arm of the X chromosome, a localization which was further refined to the interval framed by DXS7 and DXS14. We have recently shown that a novel hypervariable locus, DXS255, is very closely linked to the disease gene and is likely to be, at present, the marker closest to the disease gene. The analysis of one family, however, displayed conflicting linkage results, as all of the informative markers situated in the Xp11-q22 region appeared to recombine with the disease locus in two "phase-known" meioses. We have shown by X-inactivation studies that the segregation of the disease through three obligate carrier females in this family originates from a grandpaternal mosaicism, which accounts for the apparent recombinations. This shows that germ-line mosaicism can simulate genetic heterogeneity in linkage studies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRICH R. A., STEINBERG A. G., CAMPBELL D. C. Pedigree demonstrating a sex-linked recessive condition characterized by draining ears, eczematoid dermatitis and bloody diarrhea. Pediatrics. 1954 Feb;13(2):133–139. [PubMed] [Google Scholar]
- Arveiler B., Oberlé I., Mandel J. L. Genetic mapping of nine DNA markers in the q11----q22 region of the human X chromosome. Genomics. 1987 Sep;1(1):60–66. doi: 10.1016/0888-7543(87)90105-4. [DOI] [PubMed] [Google Scholar]
- Bakker E., Van Broeckhoven C., Bonten E. J., van de Vooren M. J., Veenema H., Van Hul W., Van Ommen G. J., Vandenberghe A., Pearson P. L. Germline mosaicism and Duchenne muscular dystrophy mutations. Nature. 1987 Oct 8;329(6139):554–556. doi: 10.1038/329554a0. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Tsipouras P., Bonadio J. F., Starman B. J., Schwartz R. C. Perinatal lethal osteogenesis imperfecta (OI type II): a biochemically heterogeneous disorder usually due to new mutations in the genes for type I collagen. Am J Hum Genet. 1988 Feb;42(2):237–248. [PMC free article] [PubMed] [Google Scholar]
- Chen J. D., Hejtmancik J. F., Romeo G., Lindlof M., Boehm C., Caskey C. T., Kress W., Fischbeck K. H., Dreier M., Serravalle S. A genetic linkage map of five marker loci in and around the Duchenne muscular dystrophy locus. Genomics. 1989 Jan;4(1):105–109. doi: 10.1016/0888-7543(89)90322-4. [DOI] [PubMed] [Google Scholar]
- Darras B. T., Blattner P., Harper J. F., Spiro A. J., Alter S., Francke U. Intragenic deletions in 21 Duchenne muscular dystrophy (DMD)/Becker muscular dystrophy (BMD) families studied with the dystrophin cDNA: location of breakpoints on HindIII and BglII exon-containing fragment maps, meiotic and mitotic origin of the mutations. Am J Hum Genet. 1988 Nov;43(5):620–629. [PMC free article] [PubMed] [Google Scholar]
- Darras B. T., Francke U. A partial deletion of the muscular dystrophy gene transmitted twice by an unaffected male. Nature. 1987 Oct 8;329(6139):556–558. doi: 10.1038/329556a0. [DOI] [PubMed] [Google Scholar]
- Davies K. E., Mandel J. L., Weissenbach J., Fellous M. Report of the committee on the genetic constitution of the X and Y chromosomes. Cytogenet Cell Genet. 1987;46(1-4):277–315. doi: 10.1159/000132481. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Kohn D. B., Winkelstein J. A., Vogelstein B., Blaese R. M. Carrier detection in the Wiskott Aldrich syndrome. Blood. 1988 Nov;72(5):1735–1739. [PubMed] [Google Scholar]
- Fraser N. J., Boyd Y., Brownlee G. G., Craig I. W. Multi-allelic RFLP for M27 beta, an anonymous single copy genomic clone at Xp11.3-Xcen [HGM9 provisional no. DXS255]. Nucleic Acids Res. 1987 Nov 25;15(22):9616–9616. doi: 10.1093/nar/15.22.9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser N. J., Boyd Y., Craig I. Isolation and characterization of a human variable copy number tandem repeat at Xcen-p11.22. Genomics. 1989 Jul;5(1):144–148. doi: 10.1016/0888-7543(89)90099-2. [DOI] [PubMed] [Google Scholar]
- Fryns J. P., Kleczkowska A., Verresen H., van den Berghe H. Germinal mosaicism in achondroplasia: a family with 3 affected siblings of normal parents. Clin Genet. 1983 Sep;24(3):156–158. doi: 10.1111/j.1399-0004.1983.tb02232.x. [DOI] [PubMed] [Google Scholar]
- Gealy W. J., Dwyer J. M., Harley J. B. Allelic exclusion of glucose-6-phosphate dehydrogenase in platelets and T lymphocytes from a Wiskott-Aldrich syndrome carrier. Lancet. 1980 Jan 12;1(8159):63–65. doi: 10.1016/s0140-6736(80)90492-4. [DOI] [PubMed] [Google Scholar]
- Gitschier J. Maternal duplication associated with gene deletion in sporadic hemophilia. Am J Hum Genet. 1988 Sep;43(3):274–279. [PMC free article] [PubMed] [Google Scholar]
- Greer W. L., Kwong P. C., Peacocke M., Ip P., Rubin L. A., Siminovitch K. A. X-chromosome inactivation in the Wiskott-Aldrich syndrome: a marker for detection of the carrier state and identification of cell lineages expressing the gene defect. Genomics. 1989 Jan;4(1):60–67. doi: 10.1016/0888-7543(89)90315-7. [DOI] [PubMed] [Google Scholar]
- Hall J. G. Review and hypotheses: somatic mosaicism: observations related to clinical genetics. Am J Hum Genet. 1988 Oct;43(4):355–363. [PMC free article] [PubMed] [Google Scholar]
- Hendriks R. W., Mensink E. J., Kraakman M. E., Thompson A., Schuurman R. K. Evidence for male X chromosomal mosaicism in X-linked agammaglobulinemia. Hum Genet. 1989 Oct;83(3):267–270. doi: 10.1007/BF00285169. [DOI] [PubMed] [Google Scholar]
- Keith D. H., Singer-Sam J., Riggs A. D. Active X chromosome DNA is unmethylated at eight CCGG sites clustered in a guanine-plus-cytosine-rich island at the 5' end of the gene for phosphoglycerate kinase. Mol Cell Biol. 1986 Nov;6(11):4122–4125. doi: 10.1128/mcb.6.11.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan S. P., Sandkuyl L. A., Blaese M., Kunkel L. M., Bruns G., Parmley R., Skarshaug S., Page D. C., Ott J., Rosen F. S. Genetic mapping of the Wiskott-Aldrich syndrome with two highly-linked polymorphic DNA markers. Genomics. 1988 Jul;3(1):39–43. doi: 10.1016/0888-7543(88)90156-5. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddalena A., Sosnoski D. M., Berry G. T., Nussbaum R. L. Mosaicism for an intragenic deletion in a boy with mild ornithine transcarbamylase deficiency. N Engl J Med. 1988 Oct 13;319(15):999–1003. doi: 10.1056/NEJM198810133191507. [DOI] [PubMed] [Google Scholar]
- Mandel J. L., Willard H. F., Nussbaum R. L., Romeo G., Puck J. M., Davies K. E. Report of the committee on the genetic constitution of the X chromosome. Cytogenet Cell Genet. 1989;51(1-4):384–437. doi: 10.1159/000132801. [DOI] [PubMed] [Google Scholar]
- Mensink E. J., Thompson A., Schot J. D., Kraakman M. E., Sandkuyl L. A., Schuurman R. K. Genetic heterogeneity in X-linked agammaglobulinemia complicates carrier detection and prenatal diagnosis. Clin Genet. 1987 Feb;31(2):91–96. doi: 10.1111/j.1399-0004.1987.tb02775.x. [DOI] [PubMed] [Google Scholar]
- Mensink E. J., Thompson A., Schot J. D., van de Greef W. M., Sandkuyl L. A., Schuurman R. K. Mapping of a gene for X-linked agammaglobulinemia and evidence for genetic heterogeneity. Hum Genet. 1986 Aug;73(4):327–332. doi: 10.1007/BF00279095. [DOI] [PubMed] [Google Scholar]
- Mentzer S. J., Remold-O'Donnell E., Crimmins M. A., Bierer B. E., Rosen F. S., Burakoff S. J. Sialophorin, a surface sialoglycoprotein defective in the Wiskott-Aldrich syndrome, is involved in human T lymphocyte proliferation. J Exp Med. 1987 May 1;165(5):1383–1392. doi: 10.1084/jem.165.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlé I., Camerino G., Kloepfer C., Moisan J. P., Grzeschik K. H., Hellkuhl B., Hors-Cayla M. C., Van Cong N., Weil D., Mandel J. L. Characterization of a set of X-linked sequences and of a panel of somatic cell hybrids useful for the regional mapping of the human X chromosome. Hum Genet. 1986 Jan;72(1):43–49. doi: 10.1007/BF00278816. [DOI] [PubMed] [Google Scholar]
- Opitz J. M. "Unstable premutation" in achondroplasia: penetrance vs phenotrance. Am J Med Genet. 1984 Oct;19(2):251–254. doi: 10.1002/ajmg.1320190207. [DOI] [PubMed] [Google Scholar]
- Pallant A., Eskenazi A., Mattei M. G., Fournier R. E., Carlsson S. R., Fukuda M., Frelinger J. G. Characterization of cDNAs encoding human leukosialin and localization of the leukosialin gene to chromosome 16. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1328–1332. doi: 10.1073/pnas.86.4.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkman R., Rappeport J., Geha R., Belli J., Cassady R., Levey R., Nathan D. G., Rosen F. S. Complete correction of the Wiskott-Aldrich syndrome by allogeneic bone-marrow transplantation. N Engl J Med. 1978 Apr 27;298(17):921–927. doi: 10.1056/NEJM197804272981701. [DOI] [PubMed] [Google Scholar]
- Parkman R., Remold-O'Donnell E., Kenney D. M., Perrine S., Rosen F. S. Surface protein abnormalities in lymphocytes and platelets from patients with Wiskott-Aldrich syndrome. Lancet. 1981 Dec 19;2(8260-61):1387–1389. doi: 10.1016/s0140-6736(81)92802-6. [DOI] [PubMed] [Google Scholar]
- Peacocke M., Siminovitch K. A. Linkage of the Wiskott-Aldrich syndrome with polymorphic DNA sequences from the human X chromosome. Proc Natl Acad Sci U S A. 1987 May;84(10):3430–3433. doi: 10.1073/pnas.84.10.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primary immunodeficiency diseases. Report prepared for the WHO by a scientific group on immunodeficiency. Clin Immunol Immunopathol. 1983 Sep;28(3):450–475. [PubMed] [Google Scholar]
- Reiser C. A., Pauli R. M., Hall J. G. Achondroplasia: unexpected familial recurrence. Am J Med Genet. 1984 Oct;19(2):245–250. doi: 10.1002/ajmg.1320190206. [DOI] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Kenney D. M., Parkman R., Cairns L., Savage B., Rosen F. S. Characterization of a human lymphocyte surface sialoglycoprotein that is defective in Wiskott-Aldrich syndrome. J Exp Med. 1984 Jun 1;159(6):1705–1723. doi: 10.1084/jem.159.6.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley C. S., Remold-O'Donnell E., Davis A. E., 3rd, Bruns G. A., Rosen F. S., Carroll M. C., Whitehead A. S. Molecular characterization of sialophorin (CD43), the lymphocyte surface sialoglycoprotein defective in Wiskott-Aldrich syndrome. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2819–2823. doi: 10.1073/pnas.86.8.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Preisinger A. C., Willard H. F., Michelson A. M., Riggs A. D., Orkin S. H. Clonal analysis using recombinant DNA probes from the X-chromosome. Cancer Res. 1987 Sep 15;47(18):4806–4813. [PubMed] [Google Scholar]
- Wood S., McGillivray B. C. Germinal mosaicism in Duchenne muscular dystrophy. Hum Genet. 1988 Mar;78(3):282–284. doi: 10.1007/BF00291677. [DOI] [PubMed] [Google Scholar]
- de Saint Basile G., Arveiler B., Fraser N. J., Boyd Y., Graig I. W., Griscelli G., Fischer A. Close linkage of hypervariable marker DXS255 to disease locus of Wiskott-Aldrich syndrome. Lancet. 1989 Dec 2;2(8675):1319–1321. doi: 10.1016/s0140-6736(89)91920-x. [DOI] [PubMed] [Google Scholar]