Abstract
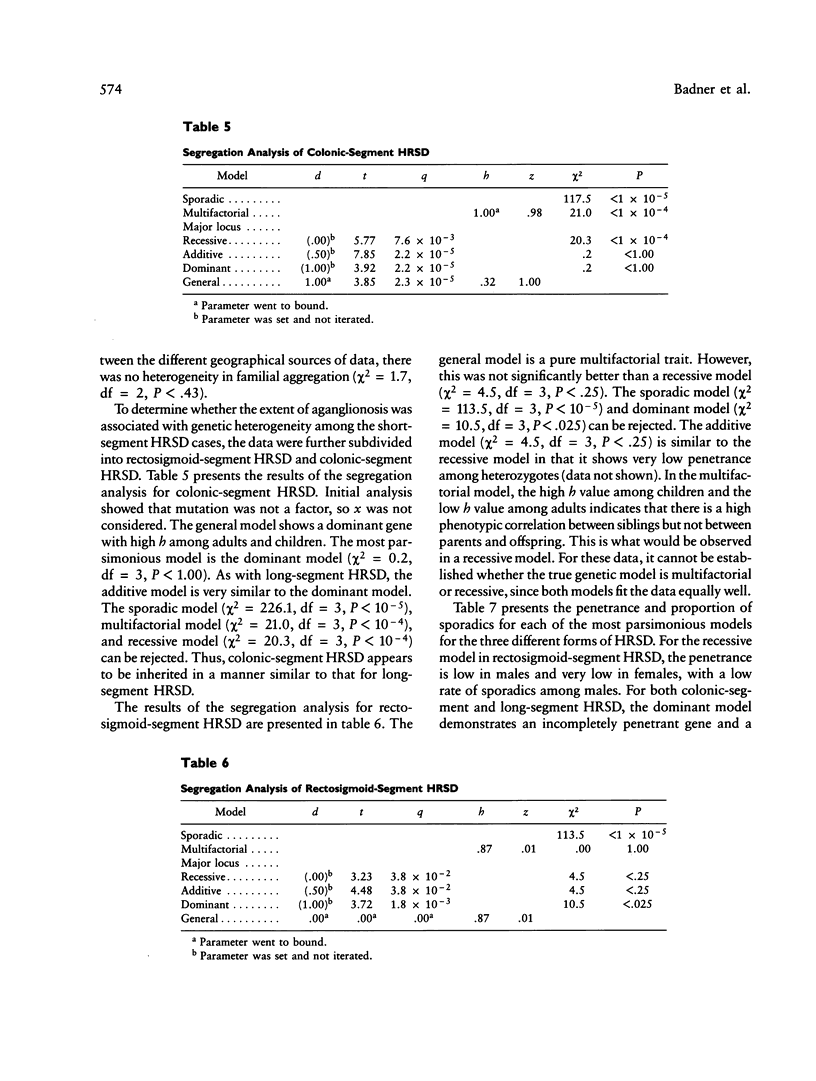
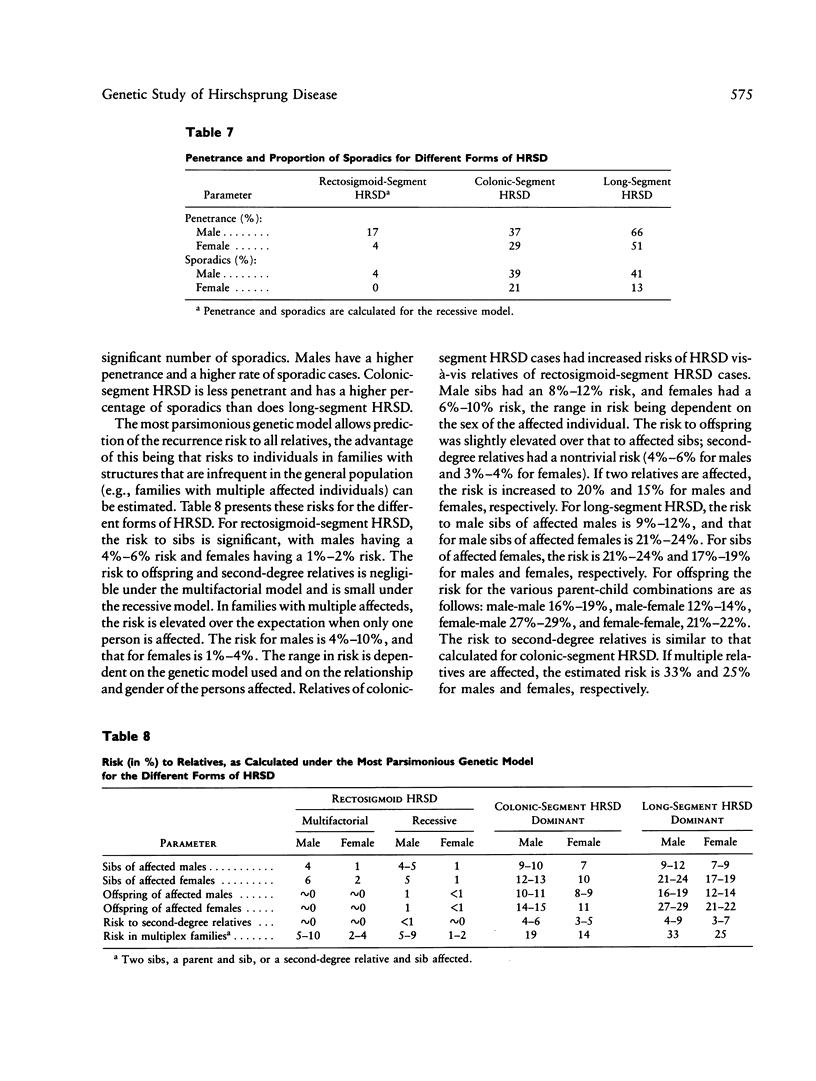
Hirschsprung disease, or congenital aganglionic megacolon, is commonly assumed to be a sex-modified multifactorial trait. To test this hypothesis, complex segregation analysis was performed on data on 487 probands and their families. Demographic information on probands and the recurrence risk to relatives of probands are presented. An increased sex ratio (3.9 male:female) and an elevated risk to sibs (4%), as compared with the population incidence (0.02%), are observed, with the sex ratio decreasing and the recurrence risk to sibs increasing as the aganglionosis becomes more extensive. Down syndrome was found at an increased frequency among affected individuals but not among their unaffected sibs, and the increase was not associated with maternal age. Complex segregation analysis was performed on these family data. The families were classified into separate categories by extent of aganglionosis. For cases with aganglionosis beyond the sigmoid colon, the mode of inheritance is compatible with a dominant gene with incomplete penetrance, while for cases with aganglionosis extending no farther than the sigmoid colon, the inheritance pattern is equally likely to be either multifactorial or due to a recessive gene with very low penetrance. A model of gene action with random effects during morphogenesis is compatible with our observations.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beedgen B., Nützenadel W., Querfeld U., Weiss-Wichert P. "Partial trisomy 22 and 11" due to a paternal 11;22 translocation associated with Hirschsprung disease. Eur J Pediatr. 1986 Aug;145(3):229–232. doi: 10.1007/BF00446075. [DOI] [PubMed] [Google Scholar]
- Besson W. T., 3rd, Kirby M. L., Van Mierop L. H., Teabeaut J. R., 2nd Effects of the size of lesions of the cardiac neural crest at various embryonic ages on incidence and type of cardiac defects. Circulation. 1986 Feb;73(2):360–364. doi: 10.1161/01.cir.73.2.360. [DOI] [PubMed] [Google Scholar]
- Carmi R., Hawley P., Wood J. W., Gerald P. S. Hirschsprung disease in progeny of affected individuals: a case report and review of the literature. Birth Defects Orig Artic Ser. 1982;18(3B):187–191. [PubMed] [Google Scholar]
- Carter C. O., Evans K., Hickman V. Children of those treated surgically for Hirschsprung's disease. J Med Genet. 1981 Apr;18(2):87–90. doi: 10.1136/jmg.18.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I. T., Gadd M. A. Hirschsprung's disease in a kindred: a possible clue to the genetics of the disease. J Pediatr Surg. 1982 Oct;17(5):632–634. doi: 10.1016/s0022-3468(82)80124-3. [DOI] [PubMed] [Google Scholar]
- Curry C. J., Carey J. C., Holland J. S., Chopra D., Fineman R., Golabi M., Sherman S., Pagon R. A., Allanson J., Shulman S. Smith-Lemli-Opitz syndrome-type II: multiple congenital anomalies with male pseudohermaphroditism and frequent early lethality. Am J Med Genet. 1987 Jan;26(1):45–57. doi: 10.1002/ajmg.1320260110. [DOI] [PubMed] [Google Scholar]
- Fabia J., Drolette M. Malformations and leukemia in children with Down's syndrome. Pediatrics. 1970 Jan;45(1):60–70. [PubMed] [Google Scholar]
- Ferencz C., Rubin J. D., McCarter R. J., Brenner J. I., Neill C. A., Perry L. W., Hepner S. I., Downing J. W. Congenital heart disease: prevalence at livebirth. The Baltimore-Washington Infant Study. Am J Epidemiol. 1985 Jan;121(1):31–36. doi: 10.1093/oxfordjournals.aje.a113979. [DOI] [PubMed] [Google Scholar]
- Ferrell R. E., Chakravarti A., Hittner H. M., Riccardi V. M. Autosomal dominant aniridia: probable linkage to acid phosphatase-1 locus on chromosome 2. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1580–1582. doi: 10.1073/pnas.77.3.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver K. L., Law J. C., Garver B. Hirschsprung disease: a genetic study. Clin Genet. 1985 Dec;28(6):503–508. doi: 10.1111/j.1399-0004.1985.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Goldberg E. L. An epidemiological study of Hirschsprung's disease. Int J Epidemiol. 1984 Dec;13(4):479–485. doi: 10.1093/ije/13.4.479. [DOI] [PubMed] [Google Scholar]
- Hall J. G. Review and hypotheses: somatic mosaicism: observations related to clinical genetics. Am J Hum Genet. 1988 Oct;43(4):355–363. [PMC free article] [PubMed] [Google Scholar]
- Hamilton J., Bodurtha J. N. Congenital central hypoventilation syndrome and Hirschsprung's disease in half sibs. J Med Genet. 1989 Apr;26(4):272–274. doi: 10.1136/jmg.26.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook E. B., Chambers G. M. Estimated rates of Down syndrome in live births by one year maternal age intervals for mothers aged 20-49 in a New York State study-implications of the risk figures for genetic counseling and cost-benefit analysis of prenatal diagnosis programs. Birth Defects Orig Artic Ser. 1977;13(3A):123–141. [PubMed] [Google Scholar]
- Hurst J. A., Markiewicz M., Kumar D., Brett E. M. Unknown syndrome: Hirschsprung's disease, microcephaly, and iris coloboma: a new syndrome of defective neuronal migration. J Med Genet. 1988 Jul;25(7):494–497. doi: 10.1136/jmg.25.7.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs-Cohen R. J., Payette R. F., Gershon M. D., Rothman T. P. Inability of neural crest cells to colonize the presumptive aganglionic bowel of ls/ls mutant mice: requirement for a permissive microenvironment. J Comp Neurol. 1987 Jan 15;255(3):425–438. doi: 10.1002/cne.902550309. [DOI] [PubMed] [Google Scholar]
- Jarmas A. L., Weaver D. D., Padilla L. M., Stecker E., Bender H. A. Hirschsprung disease: etiologic implications of unsuccessful prenatal diagnosis. Am J Med Genet. 1983 Oct;16(2):163–167. doi: 10.1002/ajmg.1320160205. [DOI] [PubMed] [Google Scholar]
- Kurnit D. M., Layton W. M., Matthysse S. Genetics, chance, and morphogenesis. Am J Hum Genet. 1987 Dec;41(6):979–995. [PMC free article] [PubMed] [Google Scholar]
- Lalouel J. M., Morton N. E. Complex segregation analysis with pointers. Hum Hered. 1981;31(5):312–321. doi: 10.1159/000153231. [DOI] [PubMed] [Google Scholar]
- Lamont M. A., Fitchett M., Dennis N. R. Interstitial deletion of distal 13q associated with Hirschsprung's disease. J Med Genet. 1989 Feb;26(2):100–104. doi: 10.1136/jmg.26.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane P. W. Association of megacolon with two recessive spotting genes in the mouse. J Hered. 1966 Jan-Feb;57(1):29–31. doi: 10.1093/oxfordjournals.jhered.a107457. [DOI] [PubMed] [Google Scholar]
- Lipson A. H., Harvey J. Three-generation transmission of Hirschsprung's disease. Clin Genet. 1987 Sep;32(3):175–178. doi: 10.1111/j.1399-0004.1987.tb03350.x. [DOI] [PubMed] [Google Scholar]
- MCKUSICK V. A., ELDRIDGE R., HOSTETLER J. A., RUANGWIT U., EGELAND J. A. DWARFISM IN THE AMISH. II. CARTILAGE-HAIR HYPOPLASIA. Bull Johns Hopkins Hosp. 1965 May;116:285–326. [PubMed] [Google Scholar]
- Magenis R. E., Sheehy R. R., Brown M. G., McDermid H. E., White B. N., Zonana J., Weleber R. Parental origin of the extra chromosome in the cat eye syndrome: evidence from heteromorphism and in situ hybridization analysis. Am J Med Genet. 1988 Jan;29(1):9–19. doi: 10.1002/ajmg.1320290103. [DOI] [PubMed] [Google Scholar]
- Mahakrishnan A., Srinivasan M. S. Piebaldness with Hirschsprung's disease. Arch Dermatol. 1980 Oct;116(10):1102–1102. [PubMed] [Google Scholar]
- Mahboubi S., Templeton J. M., Jr Association of Hirschsprung's disease and imperforate anus in a patient with "cat-eye" syndrome. A report of one case and review of the literature. Pediatr Radiol. 1984;14(6):441–442. doi: 10.1007/BF02343439. [DOI] [PubMed] [Google Scholar]
- Morton N. E., MacLean C. J. Analysis of family resemblance. 3. Complex segregation of quantitative traits. Am J Hum Genet. 1974 Jul;26(4):489–503. [PMC free article] [PubMed] [Google Scholar]
- Omenn G. S., McKusick V. A. The association of Waardenburg syndrome and Hirschsprung megacolon. Am J Med Genet. 1979;3(3):217–223. doi: 10.1002/ajmg.1320030302. [DOI] [PubMed] [Google Scholar]
- Passarge E. The genetics of Hirschsprung's disease. Evidence for heterogeneous etiology and a study of sixty-three families. N Engl J Med. 1967 Jan 19;276(3):138–143. doi: 10.1056/NEJM196701192760303. [DOI] [PubMed] [Google Scholar]
- Preier L., Hochberger O. Familiäres Megakolon. Z Kinderchir. 1981 Jan;32(1):68–73. doi: 10.1055/s-2008-1063235. [DOI] [PubMed] [Google Scholar]
- Reiss J. A., Weleber R. G., Brown M. G., Bangs C. D., Lovrien E. W., Magenis R. E. Tandem duplication of proximal 22q: a cause of cat-eye syndrome. Am J Med Genet. 1985 Jan;20(1):165–171. doi: 10.1002/ajmg.1320200120. [DOI] [PubMed] [Google Scholar]
- Reynolds J. F., Barber J. C., Alford B. A., Chandler J. G., Kelly T. E. Familial Hirschsprung's disease and type D brachydactyly: a report of four affected males in two generations. Pediatrics. 1983 Feb;71(2):246–249. [PubMed] [Google Scholar]
- Santos H., Mateus J., Leal M. J. Hirschsprung disease associated with polydactyly, unilateral renal agenesis, hypertelorism, and congenital deafness: a new autosomal recessive syndrome. J Med Genet. 1988 Mar;25(3):204–205. doi: 10.1136/jmg.25.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle A. G., Peters J., Lyon M. F., Evans E. P., Edwards J. H., Buckle V. J. Chromosome maps of man and mouse, III. Genomics. 1987 Sep;1(1):3–18. doi: 10.1016/0888-7543(87)90099-1. [DOI] [PubMed] [Google Scholar]
- Shah K. N., Dalal S. J., Desai M. P., Sheth P. N., Joshi N. C., Ambani L. M. White forelock, pigmentary disorder of irides, and long segment Hirschsprung disease: possible variant of Waardenburg syndrome. J Pediatr. 1981 Sep;99(3):432–435. doi: 10.1016/s0022-3476(81)80339-3. [DOI] [PubMed] [Google Scholar]
- Sparkes R. S., Sparkes M. C., Kalina R. E., Pagon R. A., Salk D. J., Disteche C. M. Separation of retinoblastoma and esterase D loci in a patient with sporadic retinoblastoma and del(13)(q14.1q22.3). Hum Genet. 1984;68(3):258–259. doi: 10.1007/BF00418397. [DOI] [PubMed] [Google Scholar]
- Spouge D., Baird P. A. Hirschsprung disease in a large birth cohort. Teratology. 1985 Oct;32(2):171–177. doi: 10.1002/tera.1420320204. [DOI] [PubMed] [Google Scholar]
- Verdy M., Weber A. M., Roy C. C., Morin C. L., Cadotte M., Brochu P. Hirschsprung's disease in a family with multiple endocrine neoplasia type 2. J Pediatr Gastroenterol Nutr. 1982;1(4):603–607. doi: 10.1097/00005176-198212000-00027. [DOI] [PubMed] [Google Scholar]
- Webb G. C., Keith C. G., Campbell N. T. Concurrent de novo interstitial deletion of band 2p22 and reciprocal translocation (3;7)(p21;q22). J Med Genet. 1988 Feb;25(2):125–127. doi: 10.1136/jmg.25.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster W. Embryogenesis of the enteric ganglia in normal mice and in mice that develop congenital aganglionic megacolon. J Embryol Exp Morphol. 1973 Dec;30(3):573–585. [PubMed] [Google Scholar]
- Wright T. C., Orkin R. W., Destrempes M., Kurnit D. M. Increased adhesiveness of Down syndrome fetal fibroblasts in vitro. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2426–2430. doi: 10.1073/pnas.81.8.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Gazali L. I., Donnai D., Mueller R. F. Hirschsprung's disease, hypoplastic nails, and minor dysmorphic features: a distinct autosomal recessive syndrome? J Med Genet. 1988 Nov;25(11):758–761. doi: 10.1136/jmg.25.11.758. [DOI] [PMC free article] [PubMed] [Google Scholar]



