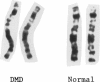Abstract
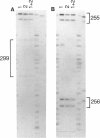
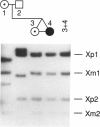
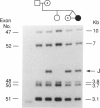
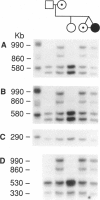
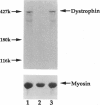
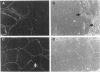
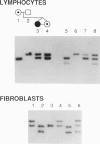
One of female MZ twins presented with muscular dystrophy. Physical examination, creatine phosphokinase levels, and muscle biopsy were consistent with Duchenne muscular dystrophy (DMD). However, because of her sex she was diagnosed as having limb-girdle muscular dystrophy. With cDNA probes to the DMD gene, a gene deletion was detected in the twins and their mother. The de novo mutation which arose in the mother was shown by novel junction fragments generated by HindIII, PstI, or TaqI when probed with cDNA8. Additional evidence of a large gene deletion was given by novel SfiI junction fragments detected by probes p20, J-Bir, and J-66 on pulsed-field gel electrophoresis (PFGE). Immunoblot analysis of muscle from the affected twin showed dystrophin of normal size but of reduced amount. Immunofluorescent visualization of dystrophin revealed foci of dystrophin-positive fibers adjacent to foci of dystrophin-negative fibers. These data indicate that the affected twin is a manifesting carrier of an abnormal DMD gene, her myopathy being a direct result of underexpression of dystrophin. Cytogenetic analysis revealed normal karyotypes, eliminating the possibility of a translocation affecting DMD gene function. Both linkage analysis and DNA fingerprint analysis revealed that each twin has two different X chromosomes, eliminating the possibility of uniparental disomy as a mechanism for DMD expression. On the basis of methylation differences of the paternal and maternal X chromosomes in these MZ twins, we propose uneven lyonization (X chromosome inactivation) as the underlying mechanism for disease expression in the affected female.
Full text
PDF

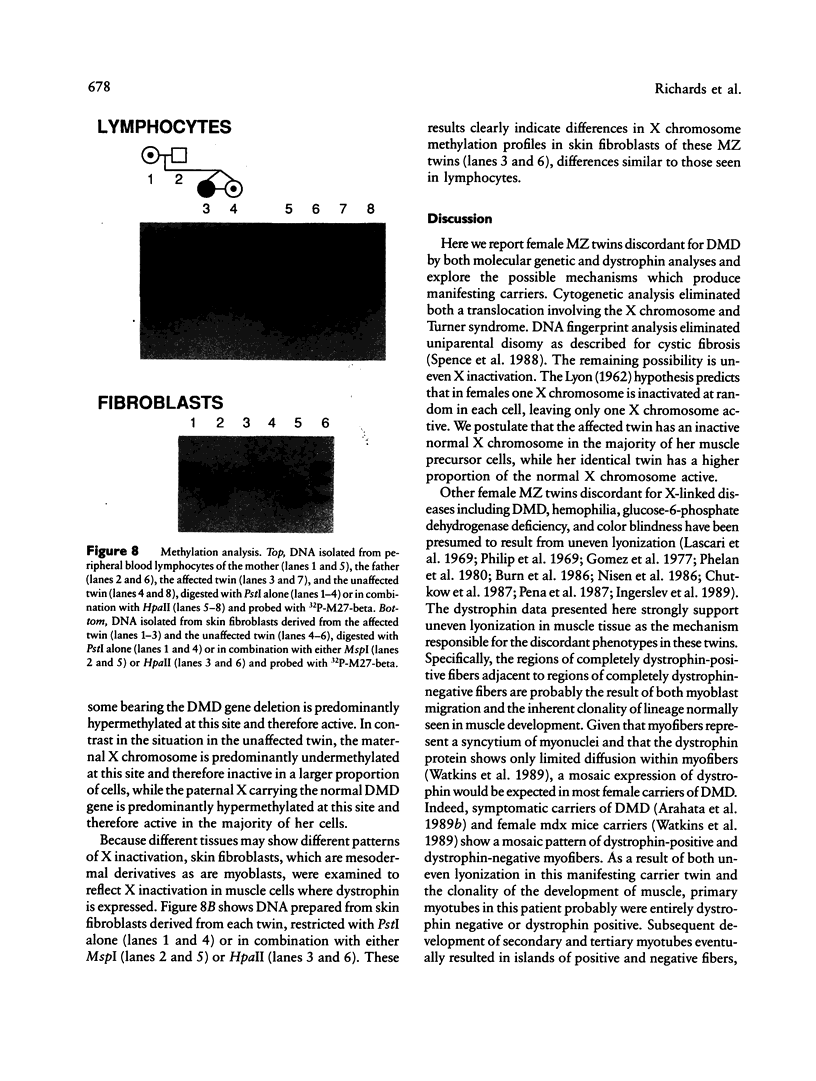



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anomalous X chromosome inactivation: the link between female zygotes, monozygotic twinning, and neural tube defects? J Med Genet. 1988 Mar;25(3):213–216. doi: 10.1136/jmg.25.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arahata K., Hoffman E. P., Kunkel L. M., Ishiura S., Tsukahara T., Ishihara T., Sunohara N., Nonaka I., Ozawa E., Sugita H. Dystrophin diagnosis: comparison of dystrophin abnormalities by immunofluorescence and immunoblot analyses. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7154–7158. doi: 10.1073/pnas.86.18.7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arahata K., Ishihara T., Kamakura K., Tsukahara T., Ishiura S., Baba C., Matsumoto T., Nonaka I., Sugita H. Mosaic expression of dystrophin in symptomatic carriers of Duchenne's muscular dystrophy. N Engl J Med. 1989 Jan 19;320(3):138–142. doi: 10.1056/NEJM198901193200302. [DOI] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Bonilla E., Samitt C. E., Miranda A. F., Hays A. P., Salviati G., DiMauro S., Kunkel L. M., Hoffman E. P., Rowland L. P. Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell. 1988 Aug 12;54(4):447–452. doi: 10.1016/0092-8674(88)90065-7. [DOI] [PubMed] [Google Scholar]
- Bonilla E., Schmidt B., Samitt C. E., Miranda A. F., Hays A. P., de Oliveira A. B., Chang H. W., Servidei S., Ricci E., Younger D. S. Normal and dystrophin-deficient muscle fibers in carriers of the gene for Duchenne muscular dystrophy. Am J Pathol. 1988 Dec;133(3):440–445. [PMC free article] [PubMed] [Google Scholar]
- Boyd Y., Munro E., Ray P., Worton R., Monaco T., Kunkel L., Craig I. Molecular heterogeneity of translocations associated with muscular dystrophy. Clin Genet. 1987 Apr;31(4):265–272. doi: 10.1111/j.1399-0004.1987.tb02805.x. [DOI] [PubMed] [Google Scholar]
- Burn J., Povey S., Boyd Y., Munro E. A., West L., Harper K., Thomas D. Duchenne muscular dystrophy in one of monozygotic twin girls. J Med Genet. 1986 Dec;23(6):494–500. doi: 10.1136/jmg.23.6.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. D., Denton M. J., Morgan G., Pearn J. H., Mackinlay A. G. The use of field-inversion gel electrophoresis for deletion detection in Duchenne muscular dystrophy. Am J Hum Genet. 1988 May;42(5):777–780. [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutkow J. G., Hyser C. L., Edwards J. A., Heffner R. R., Jr, Czyrny J. J. Monozygotic female twin carriers discordant for the clinical manifestations of Duchenne muscular dystrophy. Neurology. 1987 Jul;37(7):1147–1151. doi: 10.1212/wnl.37.7.1147. [DOI] [PubMed] [Google Scholar]
- Cullen C. R., Hubberman P., Kaslow D. C., Migeon B. R. Comparison of factor IX methylation on human active and inactive X chromosomes: implications for X inactivation and transcription of tissue-specific genes. EMBO J. 1986 Sep;5(9):2223–2229. doi: 10.1002/j.1460-2075.1986.tb04488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fraser N. J., Boyd Y., Craig I. Isolation and characterization of a human variable copy number tandem repeat at Xcen-p11.22. Genomics. 1989 Jul;5(1):144–148. doi: 10.1016/0888-7543(89)90099-2. [DOI] [PubMed] [Google Scholar]
- Gomez M. R., Engel A. G., Dewald G., Peterson H. A. Failure of inactivation of Duchenne dystrophy X-chromosome in one of female identical twins. Neurology. 1977 Jun;27(6):537–541. doi: 10.1212/wnl.27.6.537. [DOI] [PubMed] [Google Scholar]
- Grant S. G., Chapman V. M. Mechanisms of X-chromosome regulation. Annu Rev Genet. 1988;22:199–233. doi: 10.1146/annurev.ge.22.120188.001215. [DOI] [PubMed] [Google Scholar]
- Hall J. G. Review and hypotheses: somatic mosaicism: observations related to clinical genetics. Am J Hum Genet. 1988 Oct;43(4):355–363. [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. P., Fischbeck K. H., Brown R. H., Johnson M., Medori R., Loike J. D., Harris J. B., Waterston R., Brooke M., Specht L. Characterization of dystrophin in muscle-biopsy specimens from patients with Duchenne's or Becker's muscular dystrophy. N Engl J Med. 1988 May 26;318(21):1363–1368. doi: 10.1056/NEJM198805263182104. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Kunkel L. M., Angelini C., Clarke A., Johnson M., Harris J. B. Improved diagnosis of Becker muscular dystrophy by dystrophin testing. Neurology. 1989 Aug;39(8):1011–1017. doi: 10.1212/wnl.39.8.1011. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Kunkel L. M. Dystrophin abnormalities in Duchenne/Becker muscular dystrophy. Neuron. 1989 Jan;2(1):1019–1029. doi: 10.1016/0896-6273(89)90226-2. [DOI] [PubMed] [Google Scholar]
- Ingerslev J., Schwartz M., Lamm L. U., Kruse T. A., Bukh A., Stenbjerg S. Female haemophilia A in a family with seeming extreme bidirectional lyonization tendency: abnormal premature X-chromosome inactivation? Clin Genet. 1989 Jan;35(1):41–48. doi: 10.1111/j.1399-0004.1989.tb02903.x. [DOI] [PubMed] [Google Scholar]
- Keith D. H., Singer-Sam J., Riggs A. D. Active X chromosome DNA is unmethylated at eight CCGG sites clustered in a guanine-plus-cytosine-rich island at the 5' end of the gene for phosphoglycerate kinase. Mol Cell Biol. 1986 Nov;6(11):4122–4125. doi: 10.1128/mcb.6.11.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson C. M., Hoffman E. P., Kahl S. D., Kunkel L. M., Campbell K. P. Evidence for the association of dystrophin with the transverse tubular system in skeletal muscle. J Biol Chem. 1988 Jun 15;263(17):8480–8484. [PubMed] [Google Scholar]
- Koenig M., Hoffman E. P., Bertelson C. J., Monaco A. P., Feener C., Kunkel L. M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987 Jul 31;50(3):509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Kunkel L. M., Monaco A. P., Middlesworth W., Ochs H. D., Latt S. A. Specific cloning of DNA fragments absent from the DNA of a male patient with an X chromosome deletion. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4778–4782. doi: 10.1073/pnas.82.14.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYON M. F. Sex chromatin and gene action in the mammalian X-chromosome. Am J Hum Genet. 1962 Jun;14:135–148. [PMC free article] [PubMed] [Google Scholar]
- Lascari A. D., Hoak J. C., Taylor J. C. Christmas disease in a girl. Am J Dis Child. 1969 May;117(5):585–588. doi: 10.1001/archpedi.1969.02100030587016. [DOI] [PubMed] [Google Scholar]
- Lindlöf M., Kiuru A., Käriäinen H., Kalimo H., Lang H., Pihko H., Rapola J., Somer H., Somer M., Savontaus M. L. Gene deletions in X-linked muscular dystrophy. Am J Hum Genet. 1989 Apr;44(4):496–503. [PMC free article] [PubMed] [Google Scholar]
- Monaco A. P., Bertelson C. J., Middlesworth W., Colletti C. A., Aldridge J., Fischbeck K. H., Bartlett R., Pericak-Vance M. A., Roses A. D., Kunkel L. M. Detection of deletions spanning the Duchenne muscular dystrophy locus using a tightly linked DNA segment. 1985 Aug 29-Sep 4Nature. 316(6031):842–845. doi: 10.1038/316842a0. [DOI] [PubMed] [Google Scholar]
- Nisen P., Stamberg J., Ehrenpreis R., Velasco S., Shende A., Engelberg J., Karayalcin G., Waber L. The molecular basis of severe hemophilia B in a girl. N Engl J Med. 1986 Oct 30;315(18):1139–1142. doi: 10.1056/NEJM198610303151806. [DOI] [PubMed] [Google Scholar]
- Pena S. D., Karpati G., Carpenter S., Fraser F. C. The clinical consequences of X-chromosome inactivation: Duchenne muscular dystrophy in one of monozygotic twins. J Neurol Sci. 1987 Jul;79(3):337–344. doi: 10.1016/0022-510x(87)90240-1. [DOI] [PubMed] [Google Scholar]
- Philip J., Andersen C. H., Dreyer V., Freiesleben E., Gürtler H., Hauge M., Kissmeyer-Nielsen F., Nielsen L. S., Pers M., Robson E. B. Colour vision deficiency in on of two presumably monozygotic twins with secondary amenorrhoea. Ann Hum Genet. 1969 Oct;33(2):185–195. doi: 10.1111/j.1469-1809.1969.tb01644.x. [DOI] [PubMed] [Google Scholar]
- Smith D. W., Bartlett C., Harrah L. M. Monozygotic twinning and the Duhamel anomalad (imperforate anus to sirenomelia): a nonrandom association between two aberrations in morphogenesis. Birth Defects Orig Artic Ser. 1976;12(5):53–63. [PubMed] [Google Scholar]
- Spence J. E., Perciaccante R. G., Greig G. M., Willard H. F., Ledbetter D. H., Hejtmancik J. F., Pollack M. S., O'Brien W. E., Beaudet A. L. Uniparental disomy as a mechanism for human genetic disease. Am J Hum Genet. 1988 Feb;42(2):217–226. [PMC free article] [PubMed] [Google Scholar]
- Wapenaar M. C., Kievits T., Hart K. A., Abbs S., Blonden L. A., den Dunnen J. T., Grootscholten P. M., Bakker E., Verellen-Dumoulin C., Bobrow M. A deletion hot spot in the Duchenne muscular dystrophy gene. Genomics. 1988 Feb;2(2):101–108. doi: 10.1016/0888-7543(88)90090-0. [DOI] [PubMed] [Google Scholar]
- Watkins S. C., Hoffman E. P., Slayter H. S., Kunkel L. M. Immunoelectron microscopic localization of dystrophin in myofibres. Nature. 1988 Jun 30;333(6176):863–866. doi: 10.1038/333863a0. [DOI] [PubMed] [Google Scholar]
- Wolf S. F., Jolly D. J., Lunnen K. D., Friedmann T., Migeon B. R. Methylation of the hypoxanthine phosphoribosyltransferase locus on the human X chromosome: implications for X-chromosome inactivation. Proc Natl Acad Sci U S A. 1984 May;81(9):2806–2810. doi: 10.1073/pnas.81.9.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen P. H., Patel P., Chinault A. C., Mohandas T., Shapiro L. J. Differential methylation of hypoxanthine phosphoribosyltransferase genes on active and inactive human X chromosomes. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1759–1763. doi: 10.1073/pnas.81.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatz M., Passos-Bueno M. R., Rapaport D. Estimate of the proportion of Duchenne muscular dystrophy with autosomal recessive inheritance. Am J Med Genet. 1989 Mar;32(3):407–410. doi: 10.1002/ajmg.1320320328. [DOI] [PubMed] [Google Scholar]
- den Dunnen J. T., Bakker E., Breteler E. G., Pearson P. L., van Ommen G. J. Direct detection of more than 50% of the Duchenne muscular dystrophy mutations by field inversion gels. Nature. 1987 Oct 15;329(6140):640–642. doi: 10.1038/329640a0. [DOI] [PubMed] [Google Scholar]
- van Ommen G. J., Bertelson C., Ginjaar H. B., den Dunnen J. T., Bakker E., Chelly J., Matton M., van Essen A. J., Bartley J., Kunkel L. M. Long-range genomic map of the Duchenne muscular dystrophy (DMD) gene: isolation and use of J66 (DXS268), a distal intragenic marker. Genomics. 1987 Dec;1(4):329–336. doi: 10.1016/0888-7543(87)90032-2. [DOI] [PubMed] [Google Scholar]