Abstract
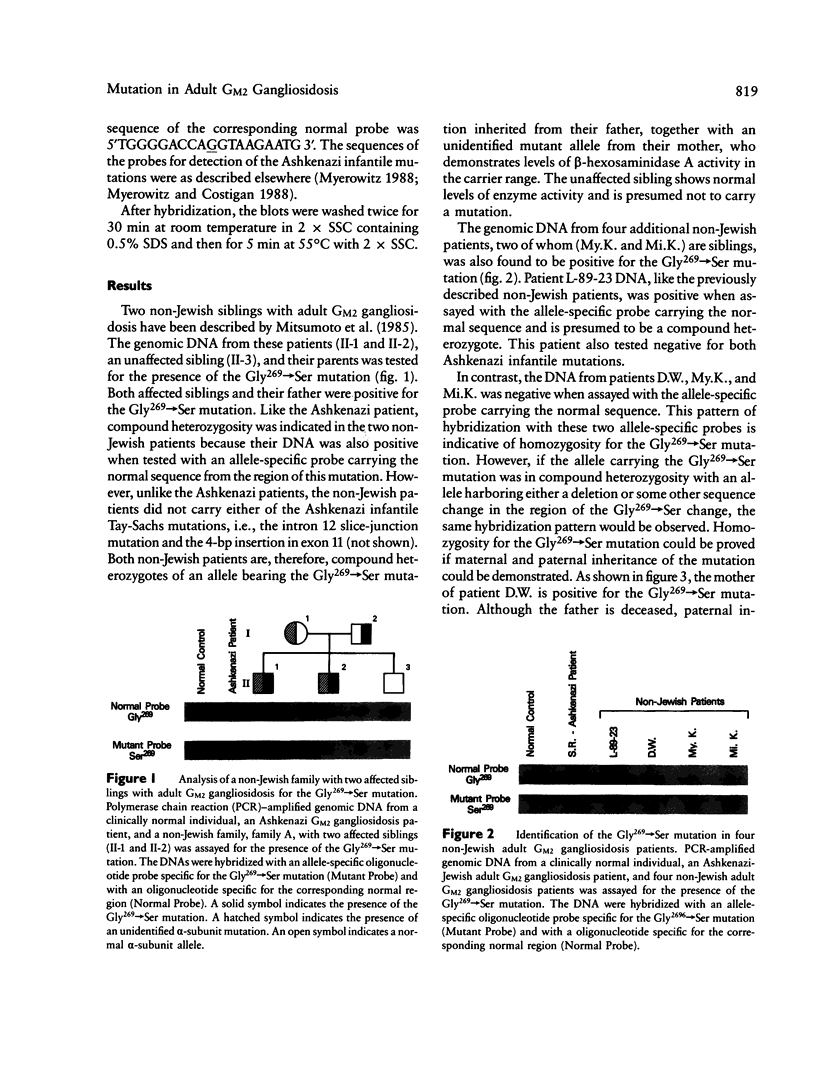
The adult form of Tay-Sachs disease, adult GM2 gangliosidosis, is an autosomal recessive neurological disorder caused by a partial deficiency of beta-hexosaminidase A. We had previously identified, in Ashkenazi-Jewish adult GM2 gangliosidosis patients, a Gly269----Ser mutation in the beta-hexosaminidase alpha-subunit. All of the Ashkenazi patients were found to be compound heterozygotes with an allele containing the Gly269----Ser mutation together with one of the Ashkenazi infantile Tay-Sachs alleles. We have now found the same Gly269----Ser mutation in six adult GM2 gangliosidosis patients from four different non-Jewish families. Genomic DNA from three of the patients, two of whom were brothers, exhibited a hybridization pattern consistent with homozygosity for the Gly269----Ser mutation. The remaining non-Jewish patients were compound heterozygotes of the Gly269----Ser mutation together with an unidentified alpha-subunit mutation. The results demonstrate that individuals homozygous for the Gly269----Ser change can be clinically affected. The same Gly269----Ser mutation in both the Ashkenazi and non-Jewish patients may be the result of a common ancestor, given that the ancestry of these non-Jewish patients, like the Ashkenazim, can be traced to eastern Europe.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arpaia E., Dumbrille-Ross A., Maler T., Neote K., Tropak M., Troxel C., Stirling J. L., Pitts J. S., Bapat B., Lamhonwah A. M. Identification of an altered splice site in Ashkenazi Tay-Sachs disease. Nature. 1988 May 5;333(6168):85–86. doi: 10.1038/333085a0. [DOI] [PubMed] [Google Scholar]
- Conzelmann E., Kytzia H. J., Navon R., Sandhoff K. Ganglioside GM2 N-acetyl-beta-D-galactosaminidase activity in cultured fibroblasts of late-infantile and adult GM2 gangliosidosis patients and of healthy probands with low hexosaminidase level. Am J Hum Genet. 1983 Sep;35(5):900–913. [PMC free article] [PubMed] [Google Scholar]
- Conzelmann E., Sandhoff K. Partial enzyme deficiencies: residual activities and the development of neurological disorders. Dev Neurosci. 1983;6(1):58–71. doi: 10.1159/000112332. [DOI] [PubMed] [Google Scholar]
- Greenberg D. A., Kaback M. M. Estimation of the frequency of hexosaminidase a variant alleles in the American Jewish population. Am J Hum Genet. 1982 May;34(3):444–451. [PMC free article] [PubMed] [Google Scholar]
- Myerowitz R., Costigan F. C. The major defect in Ashkenazi Jews with Tay-Sachs disease is an insertion in the gene for the alpha-chain of beta-hexosaminidase. J Biol Chem. 1988 Dec 15;263(35):18587–18589. [PubMed] [Google Scholar]
- Myerowitz R. Splice junction mutation in some Ashkenazi Jews with Tay-Sachs disease: evidence against a single defect within this ethnic group. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3955–3959. doi: 10.1073/pnas.85.11.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon R., Adam A. Frequency of hexosaminidase A variant alleles among Ashkenazi Jews and prenatal diagnosis of GM2 gangliosidosis. Am J Hum Genet. 1985 Sep;37(5):1031–1033. [PMC free article] [PubMed] [Google Scholar]
- Navon R., Argov Z., Frisch A. Hexosaminidase A deficiency in adults. Am J Med Genet. 1986 May;24(1):179–196. doi: 10.1002/ajmg.1320240123. [DOI] [PubMed] [Google Scholar]
- Navon R., Proia R. L. The mutations in Ashkenazi Jews with adult GM2 gangliosidosis, the adult form of Tay-Sachs disease. Science. 1989 Mar 17;243(4897):1471–1474. doi: 10.1126/science.2522679. [DOI] [PubMed] [Google Scholar]
- Neufeld E. F. Natural history and inherited disorders of a lysosomal enzyme, beta-hexosaminidase. J Biol Chem. 1989 Jul 5;264(19):10927–10930. [PubMed] [Google Scholar]
- Ohno K., Suzuki K. A splicing defect due to an exon-intron junctional mutation results in abnormal beta-hexosaminidase alpha chain mRNAs in Ashkenazi Jewish patients with Tay-Sachs disease. Biochem Biophys Res Commun. 1988 May 31;153(1):463–469. doi: 10.1016/s0006-291x(88)81247-6. [DOI] [PubMed] [Google Scholar]
- Paw B. H., Kaback M. M., Neufeld E. F. Molecular basis of adult-onset and chronic GM2 gangliosidoses in patients of Ashkenazi Jewish origin: substitution of serine for glycine at position 269 of the alpha-subunit of beta-hexosaminidase. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2413–2417. doi: 10.1073/pnas.86.7.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proia R. L., Soravia E. Organization of the gene encoding the human beta-hexosaminidase alpha-chain. J Biol Chem. 1987 Apr 25;262(12):5677–5681. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]





