Abstract
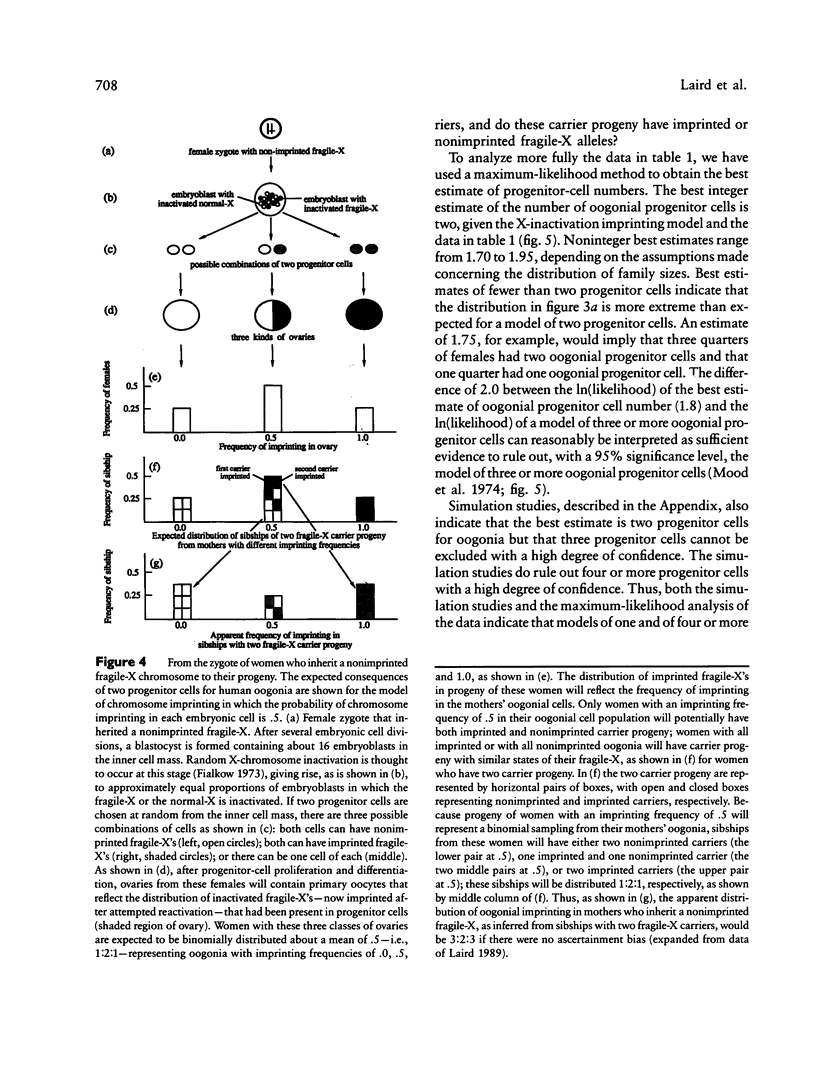
Laird has proposed that the human fragile-X syndrome is caused by abnormal chromosome imprinting. The analysis presented here supports and extends this proposal. Using published pedigrees that include DNA polymorphism (RFLP) data, we establish that the states of the fragile-X mutation termed "imprinted" and "nonimprinted" usually can be distinguished by the level of cytogenetic expression of the fragile-X chromosome. This information is then used to assess the state of the fragile-X allele in carrier progeny of individual women who inherited a nonimprinted fragile-X chromosome. From this assessment, an estimate is made of the frequency, in individual women, of primary oocytes with an imprinted fragile-X chromosome. The results of this analysis provide additional support for the specific model in which chromosome imprinting occurs in a female in, on average, half of her primary oocytes. This is the expected frequency if X-chromosome inactivation is the initial step in the imprinting of the mutant fragile-X allele. Moreover, this analysis suggests a biological explanation for peculiarities of fragile-X inheritance described by others as "clustering" and the "Sherman paradox." We interpret these peculiarities as consequences of a very small number of oogonial progenitor cells. Two progenitor cells for oogonia is the best integer estimate of the number of such cells at the time of the initial event that leads to chromosome imprinting.
Full text
PDF






















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arveiler B., Oberlé I., Vincent A., Hofker M. H., Pearson P. L., Mandel J. L. Genetic mapping of the Xq27-q28 region: new RFLP markers useful for diagnostic applications in fragile-X and hemophilia-B families. Am J Hum Genet. 1988 Feb;42(2):380–389. [PMC free article] [PubMed] [Google Scholar]
- Bakker E., Van Broeckhoven C., Bonten E. J., van de Vooren M. J., Veenema H., Van Hul W., Van Ommen G. J., Vandenberghe A., Pearson P. L. Germline mosaicism and Duchenne muscular dystrophy mutations. Nature. 1987 Oct 8;329(6139):554–556. doi: 10.1038/329554a0. [DOI] [PubMed] [Google Scholar]
- Brown W. T., Gross A. C., Chan C. B., Jenkins E. C. Genetic linkage heterogeneity in the fragile X syndrome. Hum Genet. 1985;71(1):11–18. doi: 10.1007/BF00295659. [DOI] [PubMed] [Google Scholar]
- Brown W. T., Jenkins E. C., Gross A. C., Chan C. B., Krawczun M. S., Duncan C. J., Sklower S. L., Fisch G. S. Further evidence for genetic heterogeneity in the fragile X syndrome. Hum Genet. 1987 Apr;75(4):311–321. doi: 10.1007/BF00284100. [DOI] [PubMed] [Google Scholar]
- Buchanan J. A., Buckton K. E., Gosden C. M., Newton M. S., Clayton J. F., Christie S., Hastie N. Ten families with fragile X syndrome: linkage relationships with four DNA probes from distal Xq. Hum Genet. 1987 Jun;76(2):165–172. doi: 10.1007/BF00284915. [DOI] [PubMed] [Google Scholar]
- Camerino G., Mattei M. G., Mattei J. F., Jaye M., Mandel J. L. Close linkage of fragile X-mental retardation syndrome to haemophilia B and transmission through a normal male. Nature. 1983 Dec 15;306(5944):701–704. doi: 10.1038/306701a0. [DOI] [PubMed] [Google Scholar]
- Chandra H. S., Brown S. W. Chromosome imprinting and the mammalian X chromosome. Nature. 1975 Jan 17;253(5488):165–168. doi: 10.1038/253165a0. [DOI] [PubMed] [Google Scholar]
- Crouse H V. The Controlling Element in Sex Chromosome Behavior in Sciara. Genetics. 1960 Oct;45(10):1429–1443. doi: 10.1093/genetics/45.10.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darras B. T., Francke U. A partial deletion of the muscular dystrophy gene transmitted twice by an unaffected male. Nature. 1987 Oct 8;329(6139):556–558. doi: 10.1038/329556a0. [DOI] [PubMed] [Google Scholar]
- David T. J. Dominant ectrodactyly and possible germinal mosaicism. J Med Genet. 1972 Sep;9(3):316–320. doi: 10.1136/jmg.9.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K. E., Mattei M. G., Mattei J. F., Veenema H., McGlade S., Harper K., Tommerup N., Nielsen K. B., Mikkelsen M., Beighton P. Linkage studies of X-linked mental retardation: high frequency of recombination in the telomeric region of the human X chromosome (fragile site/linkage/recombination/X chromosome). Hum Genet. 1985;70(3):249–255. doi: 10.1007/BF00273451. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J. Primordial cell pool size and lineage relationships of five human cell types. Ann Hum Genet. 1973 Jul;37(1):39–48. doi: 10.1111/j.1469-1809.1973.tb01813.x. [DOI] [PubMed] [Google Scholar]
- Fryns J. P., Kleczkowska A., Verresen H., van den Berghe H. Germinal mosaicism in achondroplasia: a family with 3 affected siblings of normal parents. Clin Genet. 1983 Sep;24(3):156–158. doi: 10.1111/j.1399-0004.1983.tb02232.x. [DOI] [PubMed] [Google Scholar]
- Gandini E., Gartler S. M., Angioni G., Argiolas N., Dell'Acqua G. Developmental implications of multiple tissue studies in glucose-6-phosphate dehydrogenase-deficient heterozygotes. Proc Natl Acad Sci U S A. 1968 Nov;61(3):945–948. doi: 10.1073/pnas.61.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartler S. M., Andina R., Gant N. Ontogeny of X-chromosome inactivation in the female germ line. Exp Cell Res. 1975 Mar 15;91(2):454–457. doi: 10.1016/0014-4827(75)90127-5. [DOI] [PubMed] [Google Scholar]
- Gartler S. M., Liskay R. M., Campbell B. K., Sparkes R., Gant N. Evidence for two functional X chromosomes in human oocytes. Cell Differ. 1972 Oct;1(4):215–218. doi: 10.1016/0045-6039(72)90039-5. [DOI] [PubMed] [Google Scholar]
- Goonewardena P., Gustavson K. H., Holmgren G., Tolun A., Chotai J., Johnsen E., Pettersson U. Analysis of fragile X-mental retardation families using flanking polymorphic DNA probes. Clin Genet. 1986 Oct;30(4):249–254. doi: 10.1111/j.1399-0004.1986.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Hall B. D. Recurrence risk in de novo 21q21q translocation Down syndrome. Am J Med Genet. 1985 Oct;22(2):417–418. doi: 10.1002/ajmg.1320220228. [DOI] [PubMed] [Google Scholar]
- Hall J. G. Review and hypotheses: somatic mosaicism: observations related to clinical genetics. Am J Hum Genet. 1988 Oct;43(4):355–363. [PMC free article] [PubMed] [Google Scholar]
- Hartl D. L. Recurrence risks for germinal mosaics. Am J Hum Genet. 1971 Mar;23(2):124–134. [PMC free article] [PubMed] [Google Scholar]
- Israel M. H. Autosomal suppressor gene for fragile-X: an hypothesis. Am J Med Genet. 1987 Jan;26(1):19–31. doi: 10.1002/ajmg.1320260106. [DOI] [PubMed] [Google Scholar]
- Johnson T. E. Analysis of pattern formation in Neurospora perithecial development using genetic mosaics. Dev Biol. 1976 Nov;54(1):23–36. doi: 10.1016/0012-1606(76)90283-9. [DOI] [PubMed] [Google Scholar]
- LYON M. F. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 1961 Apr 22;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- Laird C. D. Proposed mechanism of inheritance and expression of the human fragile-X syndrome of mental retardation. Genetics. 1987 Nov;117(3):587–599. doi: 10.1093/genetics/117.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanman J. T., Jr, Pericak-Vance M. A., Bartlett R. J., Chen J. C., Yamaoka L., Koh J., Speer M. C., Hung W. Y., Roses A. D. Familial inheritance of a DXS164 deletion mutation from a heterozygous female. Am J Hum Genet. 1987 Aug;41(2):138–144. [PMC free article] [PubMed] [Google Scholar]
- Lubs H. A. A marker X chromosome. Am J Hum Genet. 1969 May;21(3):231–244. [PMC free article] [PubMed] [Google Scholar]
- McMahon A., Fosten M., Monk M. X-chromosome inactivation mosaicism in the three germ layers and the germ line of the mouse embryo. J Embryol Exp Morphol. 1983 Apr;74:207–220. [PubMed] [Google Scholar]
- Migeon B. R., Jelalian K. Evidence for two active X chromosomes in germ cells of female before meiotic entry. Nature. 1977 Sep 15;269(5625):242–243. doi: 10.1038/269242a0. [DOI] [PubMed] [Google Scholar]
- Mintz B. Gene control of mammalian differentiation. Annu Rev Genet. 1974;8:411–470. doi: 10.1146/annurev.ge.08.120174.002211. [DOI] [PubMed] [Google Scholar]
- Nesbit M. N. X chromosome inactivation mosaicism in the mouse. Dev Biol. 1971 Oct;26(2):252–263. doi: 10.1016/0012-1606(71)90125-4. [DOI] [PubMed] [Google Scholar]
- Nussbaum R. L., Ledbetter D. H. Fragile X syndrome: a unique mutation in man. Annu Rev Genet. 1986;20:109–145. doi: 10.1146/annurev.ge.20.120186.000545. [DOI] [PubMed] [Google Scholar]
- Oberlé I., Camerino G., Wrogemann K., Arveiler B., Hanauer A., Raimondi E., Mandel J. L. Multipoint genetic mapping of the Xq26-q28 region in families with fragile X mental retardation and in normal families reveals tight linkage of markers in q26-q27. Hum Genet. 1987 Sep;77(1):60–65. doi: 10.1007/BF00284716. [DOI] [PubMed] [Google Scholar]
- Oberlé I., Heilig R., Moisan J. P., Kloepfer C., Mattéi G. M., Mattéi J. F., Boué J., Froster-Iskenius U., Jacobs P. A., Lathrop G. M. Genetic analysis of the fragile-X mental retardation syndrome with two flanking polymorphic DNA markers. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1016–1020. doi: 10.1073/pnas.83.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz J. M. On the gates of hell and a most unusual gene. Am J Med Genet. 1986 Jan-Feb;23(1-2):1–10. doi: 10.1002/ajmg.1320230102. [DOI] [PubMed] [Google Scholar]
- Pembrey M. E., Winter R. M., Davies K. E. A premutation that generates a defect at crossing over explains the inheritance of fragile X mental retardation. Am J Med Genet. 1985 Aug;21(4):709–717. doi: 10.1002/ajmg.1320210413. [DOI] [PubMed] [Google Scholar]
- REED T. E., FALLS H. F. A pedigree of aniridia with a discussion of germinal mosaicism in man. Am J Hum Genet. 1955 Mar;7(1):28–38. [PMC free article] [PubMed] [Google Scholar]
- Reik W., Collick A., Norris M. L., Barton S. C., Surani M. A. Genomic imprinting determines methylation of parental alleles in transgenic mice. Nature. 1987 Jul 16;328(6127):248–251. doi: 10.1038/328248a0. [DOI] [PubMed] [Google Scholar]
- Sapienza C., Peterson A. C., Rossant J., Balling R. Degree of methylation of transgenes is dependent on gamete of origin. Nature. 1987 Jul 16;328(6127):251–254. doi: 10.1038/328251a0. [DOI] [PubMed] [Google Scholar]
- Searle A. G. Evidence from mutable genes concerning the origin of the germ line. Basic Life Sci. 1978;12:209–224. doi: 10.1007/978-1-4684-3390-6_16. [DOI] [PubMed] [Google Scholar]
- Shapiro L. R., Wilmot P. L. Prenatal diagnosis of the fra(X) syndrome. Am J Med Genet. 1986 Jan-Feb;23(1-2):325–340. doi: 10.1002/ajmg.1320230125. [DOI] [PubMed] [Google Scholar]
- Sherman S. L., Jacobs P. A., Morton N. E., Froster-Iskenius U., Howard-Peebles P. N., Nielsen K. B., Partington M. W., Sutherland G. R., Turner G., Watson M. Further segregation analysis of the fragile X syndrome with special reference to transmitting males. Hum Genet. 1985;69(4):289–299. doi: 10.1007/BF00291644. [DOI] [PubMed] [Google Scholar]
- Sherman S. L., Morton N. E., Jacobs P. A., Turner G. The marker (X) syndrome: a cytogenetic and genetic analysis. Ann Hum Genet. 1984 Jan;48(Pt 1):21–37. doi: 10.1111/j.1469-1809.1984.tb00830.x. [DOI] [PubMed] [Google Scholar]
- Soriano P., Jaenisch R. Retroviruses as probes for mammalian development: allocation of cells to the somatic and germ cell lineages. Cell. 1986 Jul 4;46(1):19–29. doi: 10.1016/0092-8674(86)90856-1. [DOI] [PubMed] [Google Scholar]
- Steinbach P. Mental impairment in Martin-Bell syndrome is probably determined by interaction of several genes: simple explanation of phenotypic differences between unaffected and affected males with the same X chromosome. Hum Genet. 1986 Mar;72(3):248–252. doi: 10.1007/BF00291888. [DOI] [PubMed] [Google Scholar]
- Swain J. L., Stewart T. A., Leder P. Parental legacy determines methylation and expression of an autosomal transgene: a molecular mechanism for parental imprinting. Cell. 1987 Aug 28;50(5):719–727. doi: 10.1016/0092-8674(87)90330-8. [DOI] [PubMed] [Google Scholar]
- Thibodeau S. N., Dorkins H. R., Faulk K. R., Berry R., Smith A. C., Hagerman R., King A., Davies K. E. Linkage analysis using multiple DNA polymorphic markers in normal families and in families with fragile X syndrome. Hum Genet. 1988 Jul;79(3):219–227. doi: 10.1007/BF00366240. [DOI] [PubMed] [Google Scholar]
- Veenema H., Carpenter N. J., Bakker E., Hofker M. H., Ward A. M., Pearson P. L. The fragile X syndrome in a large family. III. Investigations on linkage of flanking DNA markers with the fragile site Xq27. J Med Genet. 1987 Jul;24(7):413–421. doi: 10.1136/jmg.24.7.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema H., Geraedts J. P., Beverstock G. C., Pearson P. L. The fragile X syndrome in a large family. I. Cytogenetic and clinical investigations. J Med Genet. 1987 Jan;24(1):23–31. doi: 10.1136/jmg.24.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]


