Abstract
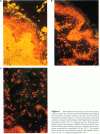
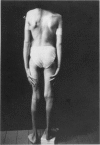
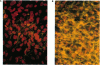
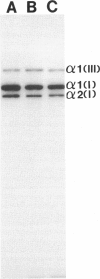
The Marfan syndrome is a dominantly inherited connective-tissue disorder characterized by ocular, cardiovascular, and musculoskeletal abnormalities. Although the underlying biochemical and molecular defect(s) of this pleiotropic disease is currently unknown, we have consistently observed apparent diminished content of elastin-associated microfibrillar fibers accumulating in skin, or produced by cultured fibroblasts, from patients with the Marfan syndrome and have documented the cosegregation of these immunofluorescent abnormalities of microfibrillar fibers with the Marfan syndrome phenotype in family studies. Recently, an unusual patient has been described with unilateral phenotypic features of the Marfan syndrome, providing an unique opportunity to compare microfibrillar fibers and other connective-tissue components between the affected and nonaffected sides. In the present report, we demonstrate striking differences in apparent content of microfibrillar fibers, as determined by indirect immunofluorescence of skin and fibroblast cultures, that are revealed when multiple homologous samples derived from different sides of the patient's body are compared. In contrast, no differences in apparent content of type III collagen or in the biosynthesis and apparent structure of types I and III (pro)collagens were found. HLA types and chromosome heteromorphisms were identical in fibroblasts from both sides of the body, eliminating the formal possibility of chimerism and suggesting that a postzygotic mutation accounts for the asymmetric manifestation of the Marfan syndrome in this patient. The observation of striking decreases in microfibrillar fibers on the affected side of the body provides further evidence that abnormalities of this component of the elastic fiber system may be central to the pathogenesis and possibly the etiology of the Marfan syndrome.
Full text
PDF


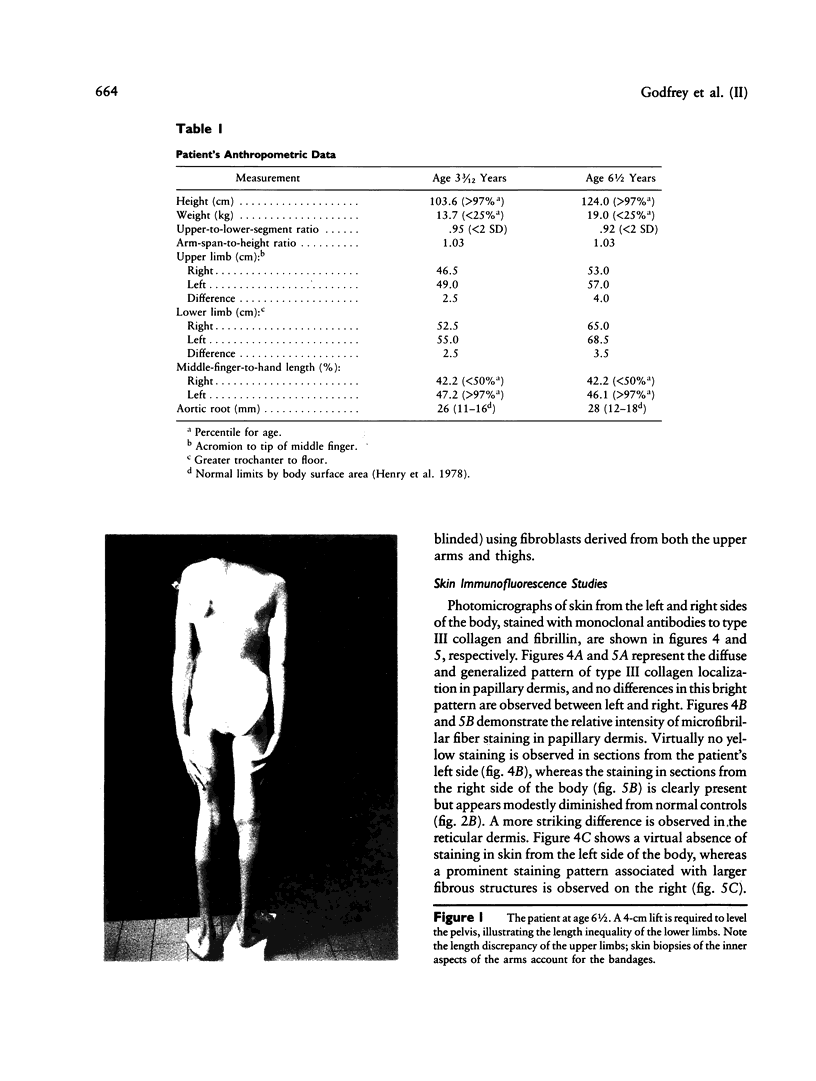
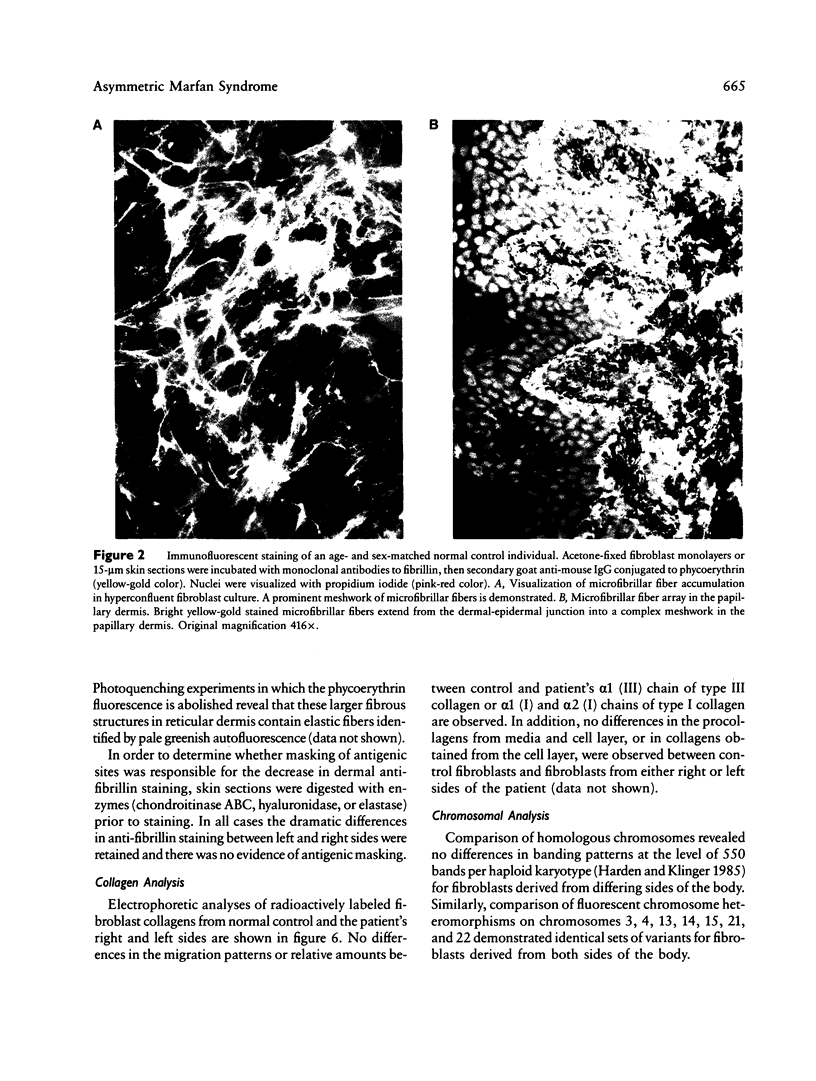
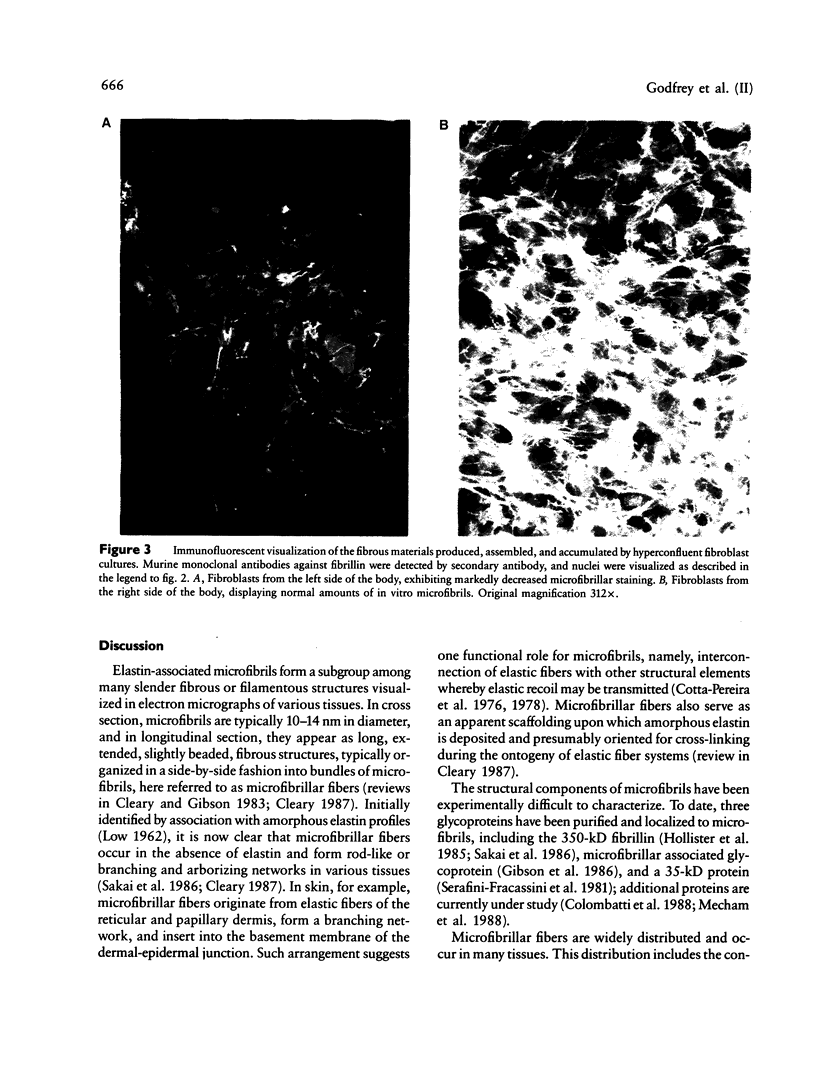
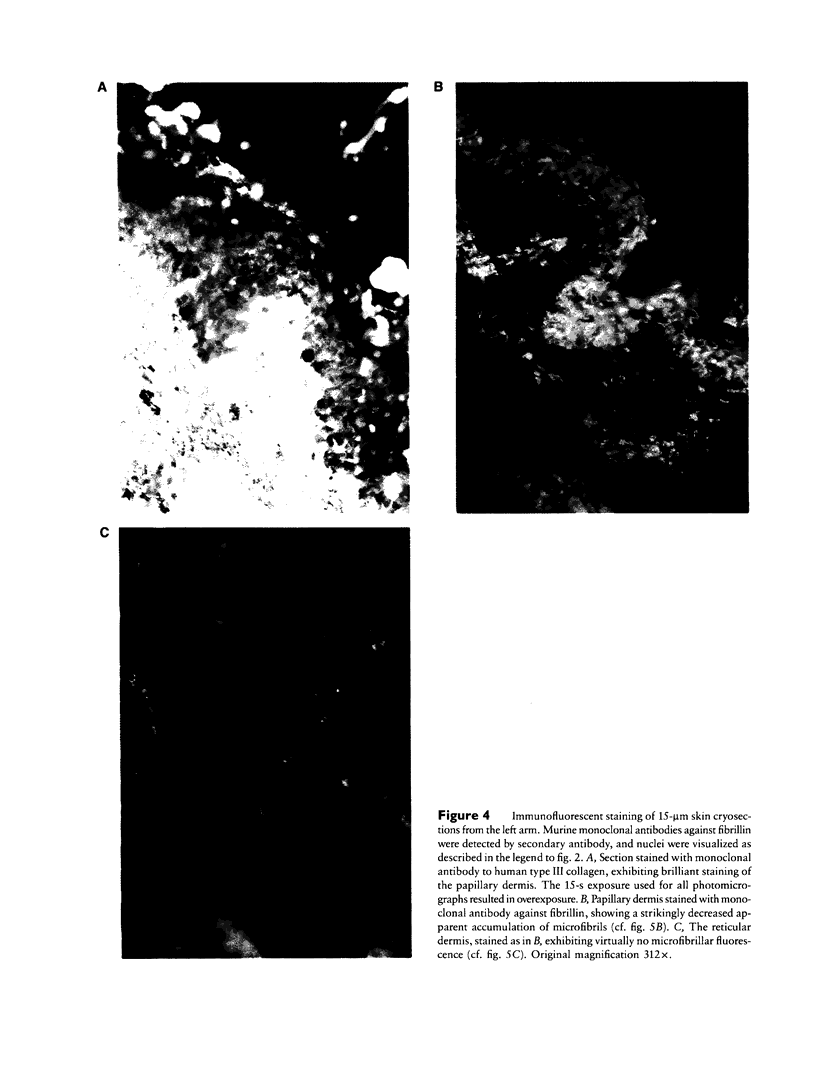
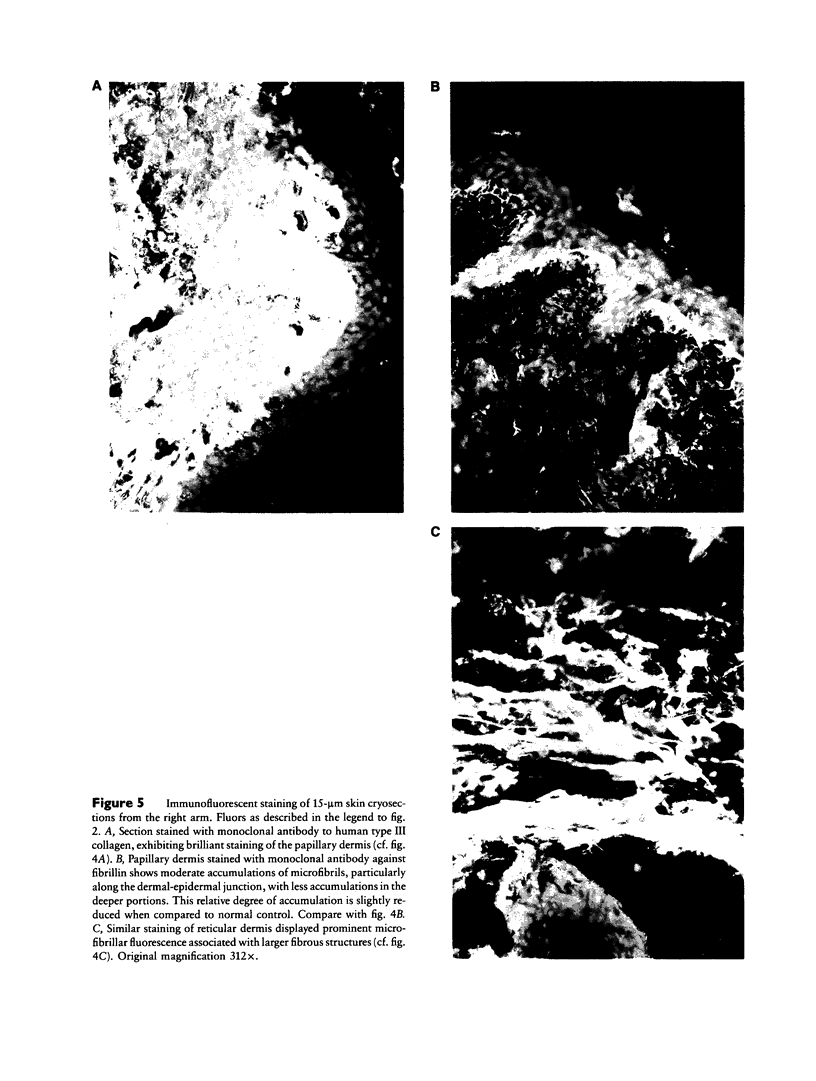



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appel A., Horwitz A. L., Dorfman A. Cell-free synthesis of hyaluronic acid in Marfan syndrome. J Biol Chem. 1979 Dec 10;254(23):12199–12203. [PubMed] [Google Scholar]
- Barsh G. S., Byers P. H. Reduced secretion of structurally abnormal type I procollagen in a form of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5142–5146. doi: 10.1073/pnas.78.8.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beighton P., de Paepe A., Danks D., Finidori G., Gedde-Dahl T., Goodman R., Hall J. G., Hollister D. W., Horton W., McKusick V. A. International Nosology of Heritable Disorders of Connective Tissue, Berlin, 1986. Am J Med Genet. 1988 Mar;29(3):581–594. doi: 10.1002/ajmg.1320290316. [DOI] [PubMed] [Google Scholar]
- Boucek R. J., Noble N. L., Gunja-Smith Z., Butler W. T. The Marfan syndrome: a deficiency in chemically stable collagen cross-links. N Engl J Med. 1981 Oct 22;305(17):988–991. doi: 10.1056/NEJM198110223051705. [DOI] [PubMed] [Google Scholar]
- Burgio R. G., Martini A., Cetta G., Zanaboni G., Vitellaro L., Danesino C. Asymmetric Marfan syndrome. Am J Med Genet. 1988 Aug;30(4):905–909. doi: 10.1002/ajmg.1320300405. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Siegel R. C., Peterson K. E., Rowe D. W., Holbrook K. A., Smith L. T., Chang Y. H., Fu J. C. Marfan syndrome: abnormal alpha 2 chain in type I collagen. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7745–7749. doi: 10.1073/pnas.78.12.7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspersson T., Lomakka G., Zech L. The 24 fluorescence patterns of the human metaphase chromosomes - distinguishing characters and variability. Hereditas. 1972;67(1):89–102. doi: 10.1111/j.1601-5223.1971.tb02363.x. [DOI] [PubMed] [Google Scholar]
- Cleary E. G., Gibson M. A. Elastin-associated microfibrils and microfibrillar proteins. Int Rev Connect Tissue Res. 1983;10:97–209. doi: 10.1016/b978-0-12-363710-9.50009-5. [DOI] [PubMed] [Google Scholar]
- Colombatti A., Bonaldo P., Volpin D., Bressan G. M. The elastin associated glycoprotein gp115. Synthesis and secretion by chick cells in culture. J Biol Chem. 1988 Nov 25;263(33):17534–17540. [PubMed] [Google Scholar]
- Cotta-Pereira G., Guerra Rodrigo F., Bittencourt-Sampaio S. Oxytalan, elaunin, and elastic fibers in the human skin. J Invest Dermatol. 1976 Mar;66(3):143–148. doi: 10.1111/1523-1747.ep12481882. [DOI] [PubMed] [Google Scholar]
- Dalgleish R., Hawkins J. R., Keston M. Exclusion of the alpha 2(I) and alpha 1(III) collagen genes as the mutant loci in a Marfan syndrome family. J Med Genet. 1987 Mar;24(3):148–151. doi: 10.1136/jmg.24.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis M. J., Sanderson M. C., Smith R. Skin collagen in idiopathic adolescent scoliosis and Marfan's syndrome. Clin Sci Mol Med. 1976 Nov;51(5):467–474. doi: 10.1042/cs0510467. [DOI] [PubMed] [Google Scholar]
- Francomano C. A., Streeten E. A., Meyers D. A., Pyeritz R. E. Marfan syndrome: exclusion of genetic linkage to three major collagen genes. Am J Med Genet. 1988 Feb;29(2):457–462. doi: 10.1002/ajmg.1320290233. [DOI] [PubMed] [Google Scholar]
- Fryns J. P., van den Berghe H. An asymmetric type of chondrodysplasia in an adult male. Another example of postzygotic mutation for an autosomal dominant gene? Clin Genet. 1986 Oct;30(4):324–327. [PubMed] [Google Scholar]
- Gibson M. A., Hughes J. L., Fanning J. C., Cleary E. G. The major antigen of elastin-associated microfibrils is a 31-kDa glycoprotein. J Biol Chem. 1986 Aug 25;261(24):11429–11436. [PubMed] [Google Scholar]
- Godfrey M., Menashe V., Weleber R. G., Koler R. D., Bigley R. H., Lovrien E., Zonana J., Hollister D. W. Cosegregation of elastin-associated microfibrillar abnormalities with the Marfan phenotype in families. Am J Hum Genet. 1990 Apr;46(4):652–660. [PMC free article] [PubMed] [Google Scholar]
- Henry W. L., Ware J., Gardin J. M., Hepner S. I., McKay J., Weiner M. Echocardiographic measurements in normal subjects. Growth-related changes that occur between infancy and early adulthood. Circulation. 1978 Feb;57(2):278–285. doi: 10.1161/01.cir.57.2.278. [DOI] [PubMed] [Google Scholar]
- LOW F. N. Microfibrils: fine filamentous components of the tissue space. Anat Rec. 1962 Feb;142:131–137. doi: 10.1002/ar.1091420205. [DOI] [PubMed] [Google Scholar]
- Laitinen O., Uitto J., Iivanainen M., Hannuksela M., Kivirikko K. I. Collagen metabolism of the skin in Marfan's syndrome. Clin Chim Acta. 1968 Sep;21(3):321–326. doi: 10.1016/0009-8981(68)90062-4. [DOI] [PubMed] [Google Scholar]
- Lamberg S. I., Dorfman A. Synthesis and degradation of hyaluronic acid in the cultured fibroblasts of Marfan's disease. J Clin Invest. 1973 Oct;52(10):2428–2433. doi: 10.1172/JCI107433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maumenee I. H. The eye in the Marfan syndrome. Trans Am Ophthalmol Soc. 1981;79:684–733. [PMC free article] [PubMed] [Google Scholar]
- Mecham R. P., Hinek A., Cleary E. G., Kucich U., Lee S. J., Rosenbloom J. Development of immunoreagents to ciliary zonules that react with protein components of elastic fiber microfibrils and with elastin-producing cells. Biochem Biophys Res Commun. 1988 Mar 15;151(2):822–826. doi: 10.1016/s0006-291x(88)80355-3. [DOI] [PubMed] [Google Scholar]
- Müller K. P., Nerlich A. G., Kunze D., Müller P. K. Studies on collagen metabolism in the Marfan syndrome. Eur J Clin Invest. 1987 Jun;17(3):218–225. doi: 10.1111/j.1365-2362.1987.tb01239.x. [DOI] [PubMed] [Google Scholar]
- Ogilvie D. J., Wordsworth B. P., Priestley L. M., Dalgleish R., Schmidtke J., Zoll B., Sykes B. C. Segregation of all four major fibrillar collagen genes in the Marfan syndrome. Am J Hum Genet. 1987 Dec;41(6):1071–1082. [PMC free article] [PubMed] [Google Scholar]
- Olson S. B., Magenis R. E., Lovrien E. W. Human chromosome variation: the discriminatory power of Q-band heteromorphism (variant) analysis in distinguishing between individuals, with specific application to cases of questionable paternity. Am J Hum Genet. 1986 Feb;38(2):235–252. [PMC free article] [PubMed] [Google Scholar]
- Overton K. M., Magenis R. E., Brady T., Chamberlin J., Parks M. Cytogenetic darkroom magic: now you see them, now you don't. Am J Hum Genet. 1976 Jul;28(4):417–419. [PMC free article] [PubMed] [Google Scholar]
- Perejda A. J., Abraham P. A., Carnes W. H., Coulson W. F., Uitto J. Marfan's syndrome: structural, biochemical, and mechanical studies of the aortic media. J Lab Clin Med. 1985 Oct;106(4):376–383. [PubMed] [Google Scholar]
- Poole A. R., Pidoux I., Reiner A., Tang L. H., Choi H., Rosenberg L. Localization of proteoglycan monomer and link protein in the matrix of bovine articular cartilage: An immunohistochemical study. J Histochem Cytochem. 1980 Jul;28(7):621–635. doi: 10.1177/28.7.6156200. [DOI] [PubMed] [Google Scholar]
- Priest R. E., Moinuddin J. F., Priest J. H. Letter: Collagen of Marfan syndrome is abnormally soluble. Nature. 1973 Oct 5;245(5423):264–266. doi: 10.1038/245264a0. [DOI] [PubMed] [Google Scholar]
- Pyeritz R. E., Fishman E. K., Bernhardt B. A., Siegelman S. S. Dural ectasia is a common feature of the Marfan syndrome. Am J Hum Genet. 1988 Nov;43(5):726–732. [PMC free article] [PubMed] [Google Scholar]
- Pyeritz R. E., McKusick V. A. The Marfan syndrome: diagnosis and management. N Engl J Med. 1979 Apr 5;300(14):772–777. doi: 10.1056/NEJM197904053001406. [DOI] [PubMed] [Google Scholar]
- Raviola G. The fine structure of the ciliary zonule and ciliary epithelium. With special regard to the organization and insertion of the zonular fibrils. Invest Ophthalmol. 1971 Nov;10(11):851–869. [PubMed] [Google Scholar]
- Rosenbloom J. Elastin: relation of protein and gene structure to disease. Lab Invest. 1984 Dec;51(6):605–623. [PubMed] [Google Scholar]
- Sakai L. Y., Keene D. R., Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986 Dec;103(6 Pt 1):2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saruk M., Eisenstein R. Aortic lesion in Marfan syndrome: the ultrastructure of cystic medial degeneration. Arch Pathol Lab Med. 1977 Feb;101(2):74–77. [PubMed] [Google Scholar]
- Serafini-Fracassini A., Ventrella G., Field M. J., Hinnie J., Onyezili N. I., Griffiths R. Characterization of a structural glycoprotein from bovine ligamentum nuchae exhibiting dual amine oxidase activity. Biochemistry. 1981 Sep 15;20(19):5424–5429. doi: 10.1021/bi00522a011. [DOI] [PubMed] [Google Scholar]
- Streeten B. W., Swann D. A., Licari P. A., Robinson M. R., Gibson S. A., Marsh N. J., Vergnes J. P., Freeman I. L. The protein composition of the ocular zonules. Invest Ophthalmol Vis Sci. 1983 Jan;24(1):119–123. [PubMed] [Google Scholar]
- Sykes B., Puddle B., Francis M., Smith R. The estimation of two collagens from human dermis by interrupted gel electrophoresis. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1472–1480. doi: 10.1016/s0006-291x(76)80180-5. [DOI] [PubMed] [Google Scholar]
- Takebayashi S., Taguchi T., Kawamura K., Sakata N. "Osmiophilic elastolysis" of peripheral organ arteries in patients with Marfan's syndrome. Acta Pathol Jpn. 1988 Nov;38(11):1433–1443. doi: 10.1111/j.1440-1827.1988.tb01085.x. [DOI] [PubMed] [Google Scholar]
- Tsipouras P., Børresen A. L., Bamforth S., Harper P. S., Berg K. Marfan syndrome: exclusion of genetic linkage to the COL1A2 gene. Clin Genet. 1986 Nov;30(5):428–432. [PubMed] [Google Scholar]