Abstract
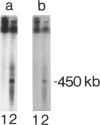
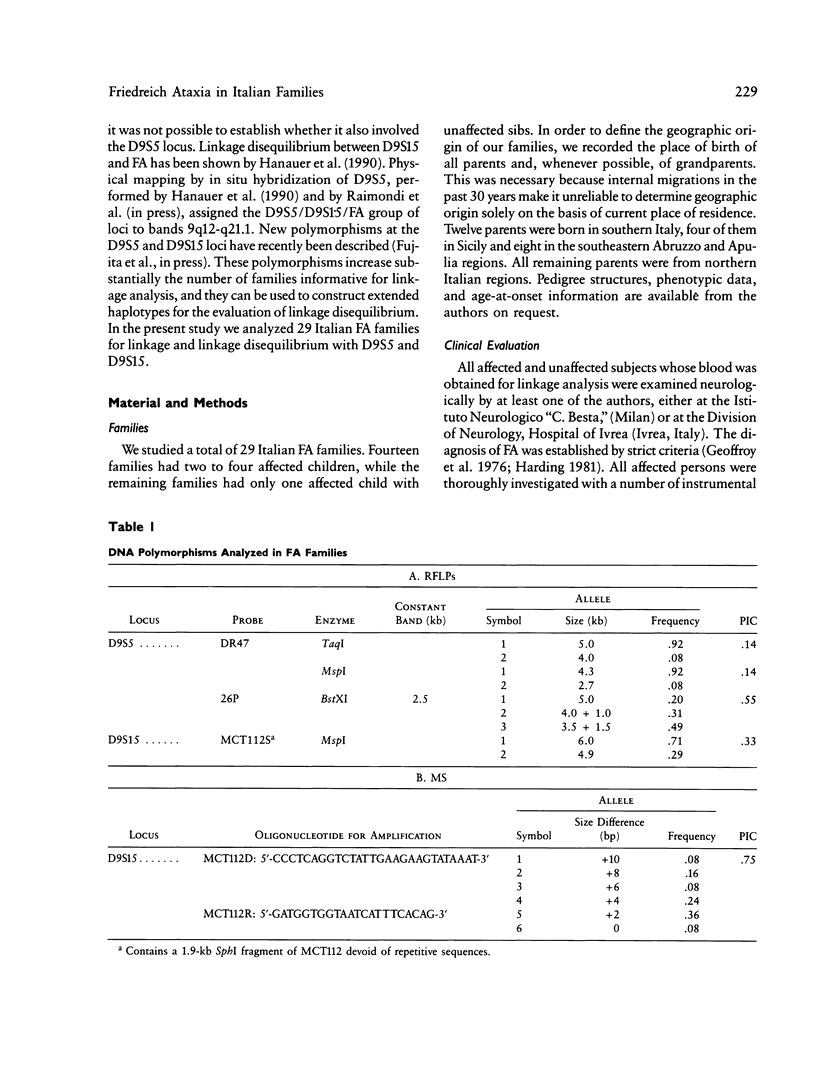
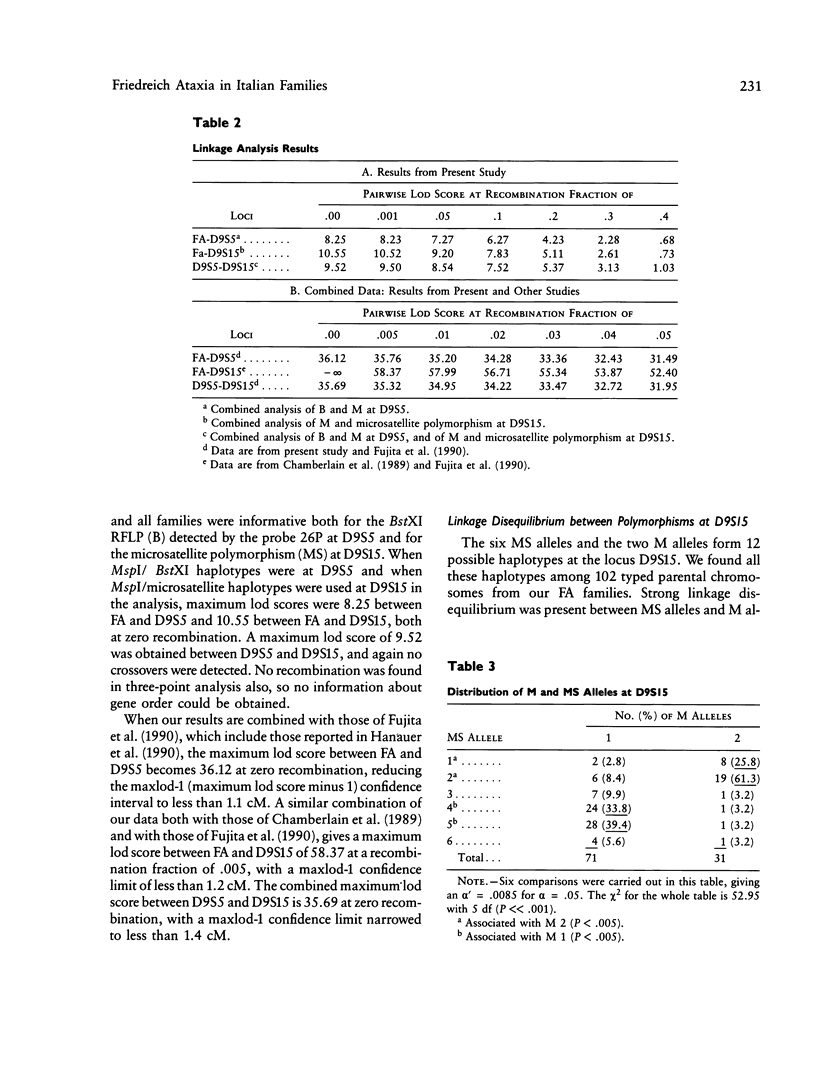
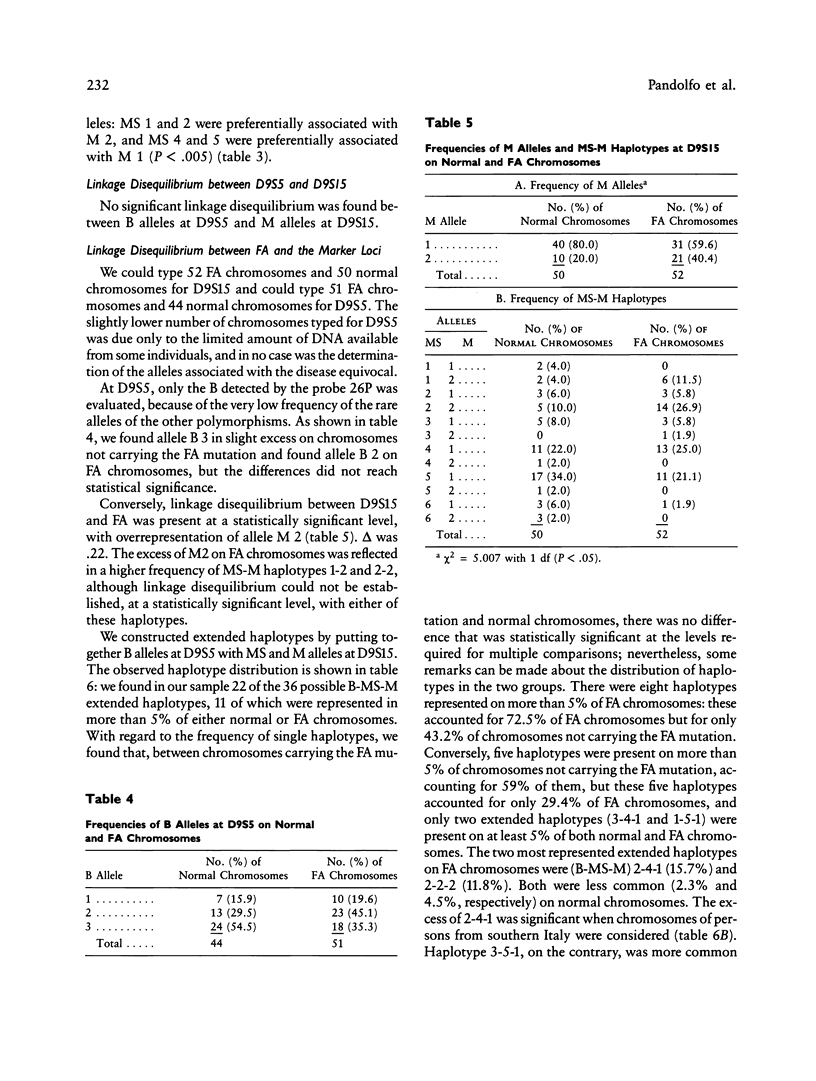
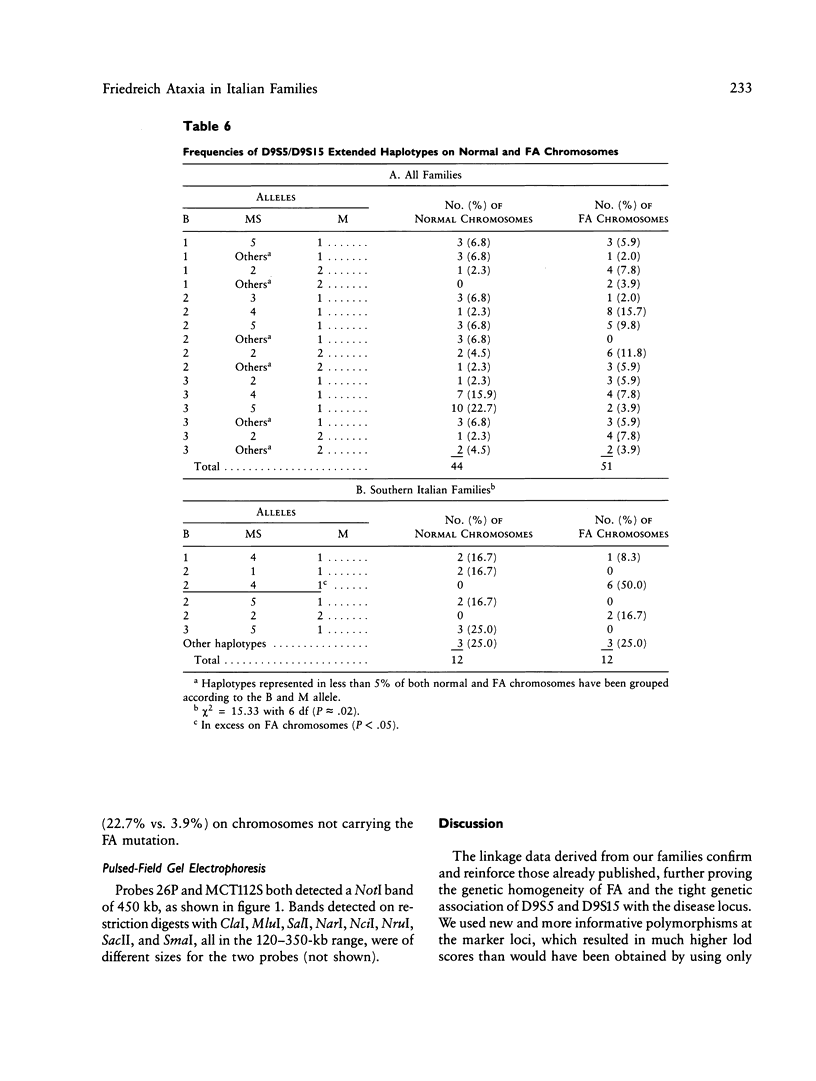
Friedreich ataxia (FA) is an autosomal recessive degenerative disease of the nervous system of unknown biochemical cause. The FA gene has been shown to be in close linkage with the two chromosome 9 markers D9S5 and D9S15, and linkage disequilibrium between FA and D9S15 has been detected in French families by Hanauer et al. We used new highly informative markers at the above loci to analyze Italian FA families for linkage and linkage disequilibrium. The new markers were a three-allele BstXI RFLP at D9S5 (PIC = .55) and a six-allele microsatellite, typed by polymerase chain reaction, at D9S15 (PIC = .75). We obtained maximum lod scores of 8.25 between FA and D9S5, 10.55 between FA and D9S15, and 9.52 between D9S5 and D9S15, all at zero recombination. Our results, combined with those reported by other authors, reduce maxlod-1 (maximum lod score minus 1) confidence limits to less than 1.1 cM between FA and D9S5, 1.2 cM between FA and D9S15, and 1.4 cM between D9S5 and D9S15. Linkage disequilibrium with FA was found only for D9S15 when all families were evaluated but was also found for a D9S5/D9S15 haplotype in a subgroup of southern Italian families. We conclude that FA, D9S5, and D9S15 are tightly clustered and that studies of geographically restricted groups may reveal a limited number of mutations responsible for the disease in the Italian population. We present preliminary evidence from pulsed-field gel electrophoresis that D9S5 and D9S15 may be less than 450 kb apart. Linkage disequilibrium between FA and D9S15 suggests that the disease gene may be at an even shorter distance from this marker locus, which therefore represents a very good starting point for cloning attempts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlson M., Nakamura Y., Krapcho K., Fujimoto E., O'Connell P., Leppert M., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence pMCT112 on chromosome 9q (D9S15). Nucleic Acids Res. 1987 Dec 23;15(24):10614–10614. doi: 10.1093/nar/15.24.10614-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti A., Buetow K. H., Antonarakis S. E., Waber P. G., Boehm C. D., Kazazian H. H. Nonuniform recombination within the human beta-globin gene cluster. Am J Hum Genet. 1984 Nov;36(6):1239–1258. [PMC free article] [PubMed] [Google Scholar]
- Chamberlain S., Shaw J., Rowland A., Wallis J., South S., Nakamura Y., von Gabain A., Farrall M., Williamson R. Mapping of mutation causing Friedreich's ataxia to human chromosome 9. Nature. 1988 Jul 21;334(6179):248–250. doi: 10.1038/334248a0. [DOI] [PubMed] [Google Scholar]
- Chamberlain S., Shaw J., Wallis J., Rowland A., Chow L., Farrall M., Keats B., Richter A., Roy M., Melancon S. Genetic homogeneity at the Friedreich ataxia locus on chromosome 9. Am J Hum Genet. 1989 Apr;44(4):518–521. [PMC free article] [PubMed] [Google Scholar]
- D'Angelo A., Di Donato S., Crenna G., Negri S., Beulche F., Uziel G., Boeri R. Friedreich's ataxia. I. Clinical, neurophysiological and in vivo biochemical studies. Ital J Neurol Sci. 1980 Oct;1(4):231–238. doi: 10.1007/BF02336703. [DOI] [PubMed] [Google Scholar]
- Fujita R., Agid Y., Trouillas P., Seck A., Tommasi-Davenas C., Driesel A. J., Olek K., Grzeschik K. H., Nakamura Y., Mandel J. L. Confirmation of linkage of Friedreich ataxia to chromosome 9 and identification of a new closely linked marker. Genomics. 1989 Jan;4(1):110–111. doi: 10.1016/0888-7543(89)90323-6. [DOI] [PubMed] [Google Scholar]
- Fujita R., Hanauer A., Sirugo G., Heilig R., Mandel J. L. Additional polymorphisms at marker loci D9S5 and D9S15 generate extended haplotypes in linkage disequilibrium with Friedreich ataxia. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1796–1800. doi: 10.1073/pnas.87.5.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy G., Barbeau A., Breton G., Lemieux B., Aube M., Leger C., Bouchard J. P. Clinical description and roentgenologic evaluation of patients with Friedreich's ataxia. Can J Neurol Sci. 1976 Nov;3(4):279–286. doi: 10.1017/s0317167100025464. [DOI] [PubMed] [Google Scholar]
- Hanauer A., Chery M., Fujita R., Driesel A. J., Gilgenkrantz S., Mandel J. L. The Friedreich ataxia gene is assigned to chromosome 9q13-q21 by mapping of tightly linked markers and shows linkage disequilibrium with D9S15. Am J Hum Genet. 1990 Jan;46(1):133–137. [PMC free article] [PubMed] [Google Scholar]
- Harding A. E. Friedreich's ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain. 1981 Sep;104(3):589–620. doi: 10.1093/brain/104.3.589. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Royle N. J., Wilson V., Wong Z. Spontaneous mutation rates to new length alleles at tandem-repetitive hypervariable loci in human DNA. Nature. 1988 Mar 17;332(6161):278–281. doi: 10.1038/332278a0. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Multilocus linkage analysis in humans: detection of linkage and estimation of recombination. Am J Hum Genet. 1985 May;37(3):482–498. [PMC free article] [PubMed] [Google Scholar]
- Leone M., Rocca W. A., Rosso M. G., Mantel N., Schoenberg B. S., Schiffer D. Friedreich's disease: survival analysis in an Italian population. Neurology. 1988 Sep;38(9):1433–1438. doi: 10.1212/wnl.38.9.1433. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzechowski H. D., Hennig J., Winter P., Grzeschik K. H., Olek K., Driesel A. J. A human single-copy DNA probe (DR 47) detects a Taq I RFLP on chromosome 9 (D9S5). Nucleic Acids Res. 1987 Aug 11;15(15):6310–6310. doi: 10.1093/nar/15.15.6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo G., Menozzi P., Ferlini A., Fadda S., Di Donato S., Uziel G., Lucci B., Capodaglio L., Filla A., Campanella G. Incidence of Friedreich ataxia in Italy estimated from consanguineous marriages. Am J Hum Genet. 1983 May;35(3):523–529. [PMC free article] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]