Abstract
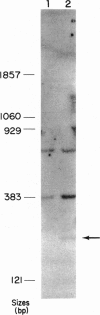
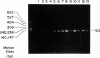
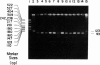
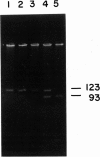
Senile systemic amyloidosis (SSA) is a late-onset disease characterized by deposition of amyloid fibrils containing transthyretin (TTR). Amino acid sequencing of protein isolated from the amyloid fibrils of a patient with SSA identified TTR containing a position - 122 isoleucine-for-valine substitution. This change led to the prediction of a genomic G-to-A transition, destroying an MaeIII restriction site. We confirmed the presence of the variant DNA fragment both by Southern blotting and by visualization of MaeIII digests of DNA amplified around codon 122, by using the polymerase chain reaction. The patient's DNA was entirely resistant to MaeIII cleavage; therefore, only the mutant sequence was present. DNA from none of either 24 controls or six other SSA patients contained the variant. Quantitative Southern blotting demonstrated that the patient's DNA contained two copies of the TTR gene per genome; the mutation was therefore homozygous rather than hemizygous. In the present case, the homozygous mutation TTR (122 Val----Ile) is associated with SSA, a finding which is consistent with autosomal recessive inheritance of this condition.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson M. D., Wallace M. R., Tejada E., Baumann H., Page B. Hereditary amyloidosis: description of a new American kindred with late onset cardiomyopathy. Appalachian amyloid. Arthritis Rheum. 1987 Feb;30(2):195–200. doi: 10.1002/art.1780300210. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Geisow M. J., Oatley S. J., Rérat B., Rérat C. Structure of prealbumin: secondary, tertiary and quaternary interactions determined by Fourier refinement at 1.8 A. J Mol Biol. 1978 May 25;121(3):339–356. doi: 10.1016/0022-2836(78)90368-6. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell G. G., 3rd, Murdoch W. L., Kyle R. A., Westermark P., Pitkänen P. Frequency and distribution of senile cardiovascular amyloid. A clinicopathologic correlation. Am J Med. 1983 Oct;75(4):618–623. doi: 10.1016/0002-9343(83)90443-6. [DOI] [PubMed] [Google Scholar]
- Cornwell G. G., 3rd, Sletten K., Johansson B., Westermark P. Evidence that the amyloid fibril protein in senile systemic amyloidosis is derived from normal prealbumin. Biochem Biophys Res Commun. 1988 Jul 29;154(2):648–653. doi: 10.1016/0006-291x(88)90188-x. [DOI] [PubMed] [Google Scholar]
- Dwulet F. E., Benson M. D. Polymorphism of human plasma thyroxine binding prealbumin. Biochem Biophys Res Commun. 1983 Jul 29;114(2):657–662. doi: 10.1016/0006-291x(83)90831-8. [DOI] [PubMed] [Google Scholar]
- FREDERIKSEN T., GOTZSCHE H., HARBOE N., KIAER W., MELLEMGAARD K. Familial primary amyloidosis with severe amyloid heart disease. Am J Med. 1962 Sep;33:328–348. doi: 10.1016/0002-9343(62)90230-9. [DOI] [PubMed] [Google Scholar]
- Ferguson R. N., Edelhoch H., Saroff H. A., Robbins J., Cahnmann H. J. Negative cooperativity in the binding of thyroxine to human serum prealbumin. Preparation of tritium-labeled 8-anilino-1-naphthalenesulfonic acid. Biochemistry. 1975 Jan 28;14(2):282–289. doi: 10.1021/bi00673a014. [DOI] [PubMed] [Google Scholar]
- Girvitz S. C., Bacchetti S., Rainbow A. J., Graham F. L. A rapid and efficient procedure for the purification of DNA from agarose gels. Anal Biochem. 1980 Aug;106(2):492–496. doi: 10.1016/0003-2697(80)90553-9. [DOI] [PubMed] [Google Scholar]
- Gitschier J., Drayna D., Tuddenham E. G., White R. L., Lawn R. M. Genetic mapping and diagnosis of haemophilia A achieved through a BclI polymorphism in the factor VIII gene. 1985 Apr 25-May 1Nature. 314(6013):738–740. doi: 10.1038/314738a0. [DOI] [PubMed] [Google Scholar]
- Gorevic P. D., Prelli F. C., Wright J., Pras M., Frangione B. Systemic senile amyloidosis. Identification of a new prealbumin (transthyretin) variant in cardiac tissue: immunologic and biochemical similarity to one form of familial amyloidotic polyneuropathy. J Clin Invest. 1989 Mar;83(3):836–843. doi: 10.1172/JCI113966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorevic P. D., Rodrigues M. M., Spencer W. H., Munoz P. C., Allen A. W., Jr, Verne A. Z. Prealbumin. A major constituent of vitreous amyloid. Ophthalmology. 1987 Jul;94(7):792–798. doi: 10.1016/s0161-6420(87)33531-6. [DOI] [PubMed] [Google Scholar]
- Gusella J. F. Location cloning strategy for characterizing genetic defects in Huntington's disease and Alzheimer's disease. FASEB J. 1989 Jul;3(9):2036–2041. doi: 10.1096/fasebj.3.9.2568302. [DOI] [PubMed] [Google Scholar]
- Hodkinson H. M., Pomerance A. The clinical significance of senile cardiac amyloidosis: a prospective clinico-pathological study. Q J Med. 1977 Jul;46(183):381–387. [PubMed] [Google Scholar]
- Holmgren G., Haettner E., Nordenson I., Sandgren O., Steen L., Lundgren E. Homozygosity for the transthyretin-met30-gene in two Swedish sibs with familial amyloidotic polyneuropathy. Clin Genet. 1988 Nov;34(5):333–338. doi: 10.1111/j.1399-0004.1988.tb02887.x. [DOI] [PubMed] [Google Scholar]
- Jacobson D. R., Santiago-Schwartz F., Buxbaum J. N. Restriction fragment analysis confirms the position 33 mutation in transthyretin from an Israeli patient (SKO) with familial amyloidotic polyneuropathy. Biochem Biophys Res Commun. 1988 May 31;153(1):198–202. doi: 10.1016/s0006-291x(88)81208-7. [DOI] [PubMed] [Google Scholar]
- Kogan S. C., Doherty M., Gitschier J. An improved method for prenatal diagnosis of genetic diseases by analysis of amplified DNA sequences. Application to hemophilia A. N Engl J Med. 1987 Oct 15;317(16):985–990. doi: 10.1056/NEJM198710153171603. [DOI] [PubMed] [Google Scholar]
- Lie J. T., Hammond P. I. Pathology of the senescent heart: anatomic observations on 237 autopsy studies of patients 90 to 105 years old. Mayo Clin Proc. 1988 Jun;63(6):552–564. doi: 10.1016/s0025-6196(12)64885-x. [DOI] [PubMed] [Google Scholar]
- Nordlie M., Sletten K., Husby G., Ranløv P. J. A new prealbumin variant in familial amyloid cardiomyopathy of Danish origin. Scand J Immunol. 1988 Jan;27(1):119–122. doi: 10.1111/j.1365-3083.1988.tb02329.x. [DOI] [PubMed] [Google Scholar]
- Olson L. J., Gertz M. A., Edwards W. D., Li C. Y., Pellikka P. A., Holmes D. R., Jr, Tajik A. J., Kyle R. A. Senile cardiac amyloidosis with myocardial dysfunction. Diagnosis by endomyocardial biopsy and immunohistochemistry. N Engl J Med. 1987 Sep 17;317(12):738–742. doi: 10.1056/NEJM198709173171205. [DOI] [PubMed] [Google Scholar]
- Paw B. H., Kaback M. M., Neufeld E. F. Molecular basis of adult-onset and chronic GM2 gangliosidoses in patients of Ashkenazi Jewish origin: substitution of serine for glycine at position 269 of the alpha-subunit of beta-hexosaminidase. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2413–2417. doi: 10.1073/pnas.86.7.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen P., Westermark P., Cornwell G. G., 3rd Senile systemic amyloidosis. Am J Pathol. 1984 Dec;117(3):391–399. [PMC free article] [PubMed] [Google Scholar]
- Sandgren O., Holmgren G., Lundgren E., Steen L. Restriction fragment length polymorphism analysis of mutated transthyretin in vitreous amyloidosis. Arch Ophthalmol. 1988 Jun;106(6):790–792. doi: 10.1001/archopht.1988.01060130860040. [DOI] [PubMed] [Google Scholar]
- Saraiva M. J., Birken S., Costa P. P., Goodman D. S. Amyloid fibril protein in familial amyloidotic polyneuropathy, Portuguese type. Definition of molecular abnormality in transthyretin (prealbumin). J Clin Invest. 1984 Jul;74(1):104–119. doi: 10.1172/JCI111390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H., Yoshioka N., Takagi Y., Sakaki Y. Structure of the chromosomal gene for human serum prealbumin. Gene. 1985;37(1-3):191–197. doi: 10.1016/0378-1119(85)90272-0. [DOI] [PubMed] [Google Scholar]
- Sletten K., Westermark P., Natvig J. B. Senile cardiac amyloid is related to prealbumin. Scand J Immunol. 1980;12(6):503–506. doi: 10.1111/j.1365-3083.1980.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Smith R. R., Hutchins G. M., Moore G. W., Humphrey R. L. Type and distribution of pulmonary parenchymal and vascular amyloid. Correlation with cardiac amyloid. Am J Med. 1979 Jan;66(1):96–104. doi: 10.1016/0002-9343(79)90488-1. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Kyle R. A., Lie J. T. Clinical significance of histopathologic patterns of cardiac amyloidosis. Mayo Clin Proc. 1984 Aug;59(8):547–555. doi: 10.1016/s0025-6196(12)61493-1. [DOI] [PubMed] [Google Scholar]
- Tawara S., Nakazato M., Kangawa K., Matsuo H., Araki S. Identification of amyloid prealbumin variant in familial amyloidotic polyneuropathy (Japanese type). Biochem Biophys Res Commun. 1983 Nov 15;116(3):880–888. doi: 10.1016/s0006-291x(83)80224-1. [DOI] [PubMed] [Google Scholar]
- Tsuzuki T., Mita S., Maeda S., Araki S., Shimada K. Structure of the human prealbumin gene. J Biol Chem. 1985 Oct 5;260(22):12224–12227. [PubMed] [Google Scholar]
- Wallace M. R., Dwulet F. E., Conneally P. M., Benson M. D. Biochemical and molecular genetic characterization of a new variant prealbumin associated with hereditary amyloidosis. J Clin Invest. 1986 Jul;78(1):6–12. doi: 10.1172/JCI112573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M. R., Dwulet F. E., Williams E. C., Conneally P. M., Benson M. D. Identification of a new hereditary amyloidosis prealbumin variant, Tyr-77, and detection of the gene by DNA analysis. J Clin Invest. 1988 Jan;81(1):189–193. doi: 10.1172/JCI113293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P., Johansson B., Natvig J. B. Senile cardiac amyloidosis: evidence of two different amyloid substances in the ageing heart. Scand J Immunol. 1979;10(4):303–308. doi: 10.1111/j.1365-3083.1979.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Wright J. R., Calkins E., Breen W. J., Stolte G., Schultz R. T. Relationship of amyloid to aging. Review of the literature and systematic study of 83 patients derived rom a general hospital population. Medicine (Baltimore) 1969 Jan;48(1):39–60. [PubMed] [Google Scholar]
- van Jaarsveld P. P., Edelhoch H., Goodman D. S., Robbins J. The interaction of human plasma retinol-binding protein and prealbumin. J Biol Chem. 1973 Jul 10;248(13):4698–4705. [PubMed] [Google Scholar]