Abstract
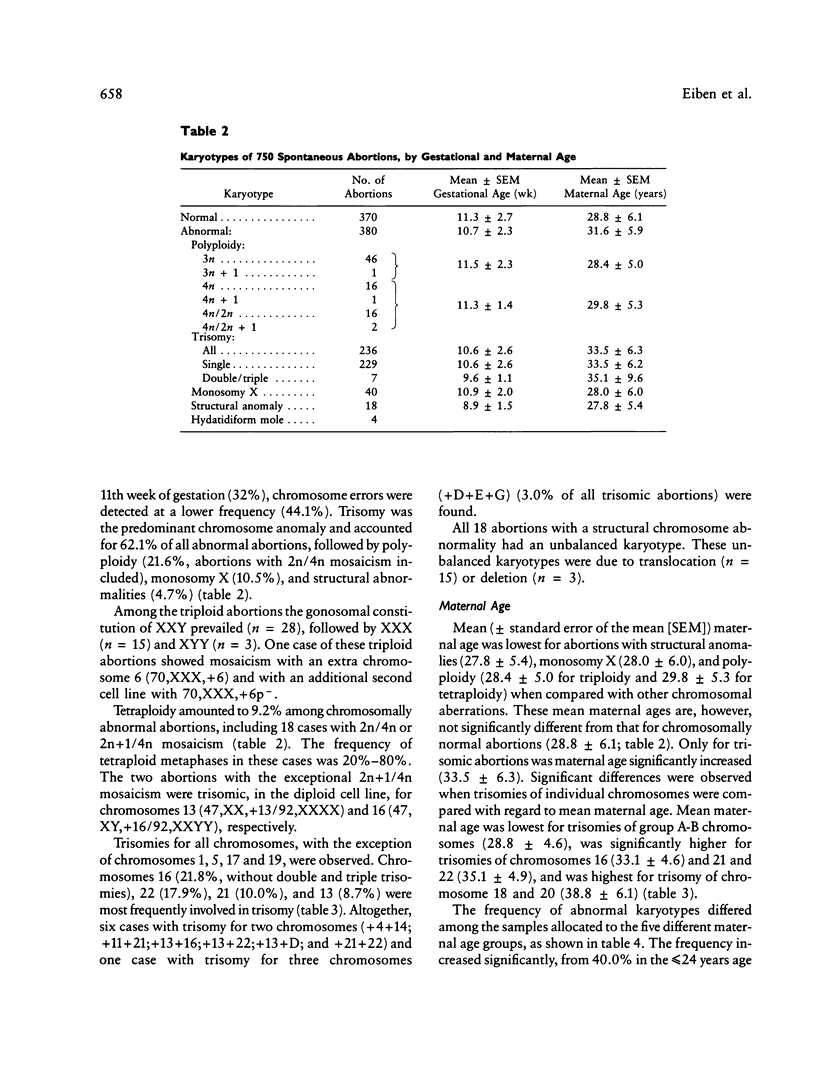
Altogether, 750 cases of spontaneous abortion between the fifth and 25th week of gestation were analyzed cytogenetically by the direct-preparation method using chorionic villi. The majority of cases (68%) were derived from early abortions before the 12th week of gestation. The frequency of abnormal karyotypes was 50.1%; trisomy was predominant (62.1%), followed by triploidy (12.4%), monosomy X (10.5%), tetraploidy (9.2%), and structural chromosome anomalies (4.7%). Among trisomies, chromosomes 16 (21.8%), 22 (17.9%), and 21 (10.0%) were prevalent. The frequency of chromosomally abnormal abortions increased with maternal age but only because of an increase of trisomy. Polyploidy and monosomy X, however, decreased. Mean maternal age was significantly increased for trisomies 16, 21, and 22 and was highest for trisomies 18 and 20. The results obtained are within the range of variability reported earlier from tissue culture–type studies. A consistent feature during our study is the excess of females in chromosomally normal abortions (male:female sex ratio 0.71). According to the methodology applied, maternal cell contamination and undetected 46,XX molar samples cannot have influenced the sex ratio. However, a bias introduced by social status or maternal age cannot be excluded. With the more rapid and convenient direct preparation of chorionic villi, reliable cytogenetic data on causes of spontaneous abortions can be obtained.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adinolfi M. Recurrent habitual abortion, HLA sharing and deliberate immunization with partner's cells: a controversial topic. Hum Reprod. 1986 Jan;1(1):45–48. doi: 10.1093/oxfordjournals.humrep.a136342. [DOI] [PubMed] [Google Scholar]
- Bartels I., Hansmann I., Eiben B. Excess of females in chromosomally normal spontaneous abortuses. Am J Med Genet. 1990 Feb;35(2):297–298. doi: 10.1002/ajmg.1320350235. [DOI] [PubMed] [Google Scholar]
- CARR D. H. CHROMOSOME STUDIES IN ABORTUSES AND STILLBORN INFANTS. Lancet. 1963 Sep 21;2(7308):603–606. doi: 10.1016/s0140-6736(63)90396-9. [DOI] [PubMed] [Google Scholar]
- Creasy M. R., Crolla J. A., Alberman E. D. A cytogenetic study of human spontaneous abortions using banding techniques. Hum Genet. 1976 Feb 29;31(2):177–196. doi: 10.1007/BF00296145. [DOI] [PubMed] [Google Scholar]
- Eiben B., Borgmann S., Schübbe I., Hansmann I. A cytogenetic study directly from chorionic villi of 140 spontaneous abortions. Hum Genet. 1987 Oct;77(2):137–141. doi: 10.1007/BF00272380. [DOI] [PubMed] [Google Scholar]
- Eiben B., Schübbe I., Borgmann S., Hansmann I. Rapid cytogenetic diagnosis of early spontaneous abortions. Lancet. 1986 May 31;1(8492):1273–1274. doi: 10.1016/s0140-6736(86)91411-x. [DOI] [PubMed] [Google Scholar]
- Hansmann I., Bartels I., Schübbe I. Cytogenetic analysis of early human abortuses after preparation of chromosomes directly from chorionic villi. Hum Genet. 1986 Feb;72(2):189–189. doi: 10.1007/BF00283947. [DOI] [PubMed] [Google Scholar]
- Hassold T. J., Jacobs P. A. Trisomy in man. Annu Rev Genet. 1984;18:69–97. doi: 10.1146/annurev.ge.18.120184.000441. [DOI] [PubMed] [Google Scholar]
- Hassold T., Chen N., Funkhouser J., Jooss T., Manuel B., Matsuura J., Matsuyama A., Wilson C., Yamane J. A., Jacobs P. A. A cytogenetic study of 1000 spontaneous abortions. Ann Hum Genet. 1980 Oct;44(Pt 2):151–178. doi: 10.1111/j.1469-1809.1980.tb00955.x. [DOI] [PubMed] [Google Scholar]
- Hassold T., Chiu D. Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet. 1985;70(1):11–17. doi: 10.1007/BF00389450. [DOI] [PubMed] [Google Scholar]
- Hassold T., MacLean C. Temporal changes in chromosome abnormality rate in human spontaneous abortions: evidence for an association between sex-chromosome monosomy and trisomy 16. Cytogenet Cell Genet. 1984;38(3):200–205. doi: 10.1159/000132060. [DOI] [PubMed] [Google Scholar]
- Hassold T., Quillen S. D., Yamane J. A. Sex ratio in spontaneous abortions. Ann Hum Genet. 1983 Jan;47(Pt 1):39–47. doi: 10.1111/j.1469-1809.1983.tb00968.x. [DOI] [PubMed] [Google Scholar]
- Hunt P. A., Jacobs P. A. In vitro growth and chromosome constitution of placental cells. I. Spontaneous and elective abortions. Cytogenet Cell Genet. 1985;39(1):1–6. doi: 10.1159/000132095. [DOI] [PubMed] [Google Scholar]
- Kajii T., Ferrier A., Niikawa N., Takahara H., Ohama K., Avirachan S. Anatomic and chromosomal anomalies in 639 spontaneous abortuses. Hum Genet. 1980;55(1):87–98. doi: 10.1007/BF00329132. [DOI] [PubMed] [Google Scholar]
- Kalousek D. K., Barrett I. J., McGillivray B. C. Placental mosaicism and intrauterine survival of trisomies 13 and 18. Am J Hum Genet. 1989 Mar;44(3):338–343. [PMC free article] [PubMed] [Google Scholar]
- Kalousek D. K., Dill F. J. Chromosomal mosaicism confined to the placenta in human conceptions. Science. 1983 Aug 12;221(4611):665–667. doi: 10.1126/science.6867735. [DOI] [PubMed] [Google Scholar]
- Machin G. A., Crolla J. A. Chromosome constitution of 500 infants dying during the perinatal period. With an appendix concerning other genetic disorders among these infants. Humangenetik. 1974;23(3):183–198. doi: 10.1007/BF00285104. [DOI] [PubMed] [Google Scholar]
- Minguillon C., Eiben B., Bähr-Porsch S., Vogel M., Hansmann I. The predictive value of chorionic villus histology for identifying chromosomally normal and abnormal spontaneous abortions. Hum Genet. 1989 Jul;82(4):373–376. doi: 10.1007/BF00274001. [DOI] [PubMed] [Google Scholar]
- Morton N. E., Chiu D., Holland C., Jacobs P. A., Pettay D. Chromosome anomalies as predictors of recurrence risk for spontaneous abortion. Am J Med Genet. 1987 Oct;28(2):353–360. doi: 10.1002/ajmg.1320280213. [DOI] [PubMed] [Google Scholar]
- Mowbray J. F., Underwood J. L., Michel M., Forbes P. B., Beard R. W. Immunisation with paternal lymphocytes in women with recurrent miscarriage. Lancet. 1987 Sep 19;2(8560):679–680. doi: 10.1016/s0140-6736(87)92457-3. [DOI] [PubMed] [Google Scholar]
- Rehder H., Coerdt W., Eggers R., Klink F., Schwinger E. Is there a correlation between morphological and cytogenetic findings in placental tissue from early missed abortions? Hum Genet. 1989 Jul;82(4):377–385. doi: 10.1007/BF00274002. [DOI] [PubMed] [Google Scholar]
- Rossant J., Joyner A. L. Towards a molecular-genetic analysis of mammalian development. Trends Genet. 1989 Aug;5(8):277–283. doi: 10.1016/0168-9525(89)90102-9. [DOI] [PubMed] [Google Scholar]
- Searle A. G., Beechey C. V. Noncomplementation phenomena and their bearing on nondisjunctional effects. Basic Life Sci. 1985;36:363–376. doi: 10.1007/978-1-4613-2127-9_25. [DOI] [PubMed] [Google Scholar]
- Short R. V. When a conception fails to become a pregnancy. Ciba Found Symp. 1978;(64):377–394. doi: 10.1002/9780470720479.ch16. [DOI] [PubMed] [Google Scholar]
- Simoni G., Brambati B., Danesino C., Rossella F., Terzoli G. L., Ferrari M., Fraccaro M. Efficient direct chromosome analyses and enzyme determinations from chorionic villi samples in the first trimester of pregnancy. Hum Genet. 1983;63(4):349–357. doi: 10.1007/BF00274761. [DOI] [PubMed] [Google Scholar]
- Sutherland G. R., Carter R. F., Bauld R., Smith I. I., Bain A. D. Chromosome studies at the paediatric necropsy. Ann Hum Genet. 1978 Oct;42(2):173–181. doi: 10.1111/j.1469-1809.1978.tb00647.x. [DOI] [PubMed] [Google Scholar]
- Thomas M. L., Harger J. H., Wagener D. K., Rabin B. S., Gill T. J., 3rd HLA sharing and spontaneous abortion in humans. Am J Obstet Gynecol. 1985 Apr 15;151(8):1053–1058. doi: 10.1016/0002-9378(85)90379-5. [DOI] [PubMed] [Google Scholar]
- Visaria P. M. Sex ratio at birth in territories with a relatively complete registration. Eugen Q. 1967 Jun;14(2):132–142. doi: 10.1080/19485565.1967.9987713. [DOI] [PubMed] [Google Scholar]
- Warburton D., Kline J., Stein Z., Hutzler M., Chin A., Hassold T. Does the karyotype of a spontaneous abortion predict the karyotype of a subsequent abortion? Evidence from 273 women with two karyotyped spontaneous abortions. Am J Hum Genet. 1987 Sep;41(3):465–483. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Ito T., Watanabe M., Watanabe G. Causes of chromosome anomalies suggested by cytogenetic epidemiology of induced abortions. Hum Genet. 1982;60(4):360–364. doi: 10.1007/BF00569219. [DOI] [PubMed] [Google Scholar]
- Zhou X. T., Tong H. S., Wong S. G., Shen Q. E., Fu X. W., Cui Y. Q. Chromosome abnormalities in early pregnancy analyzed by direct chromosome preparation of chorionic villi. Hum Genet. 1989 Oct;83(3):277–279. doi: 10.1007/BF00285172. [DOI] [PubMed] [Google Scholar]


