Abstract
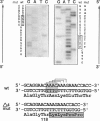
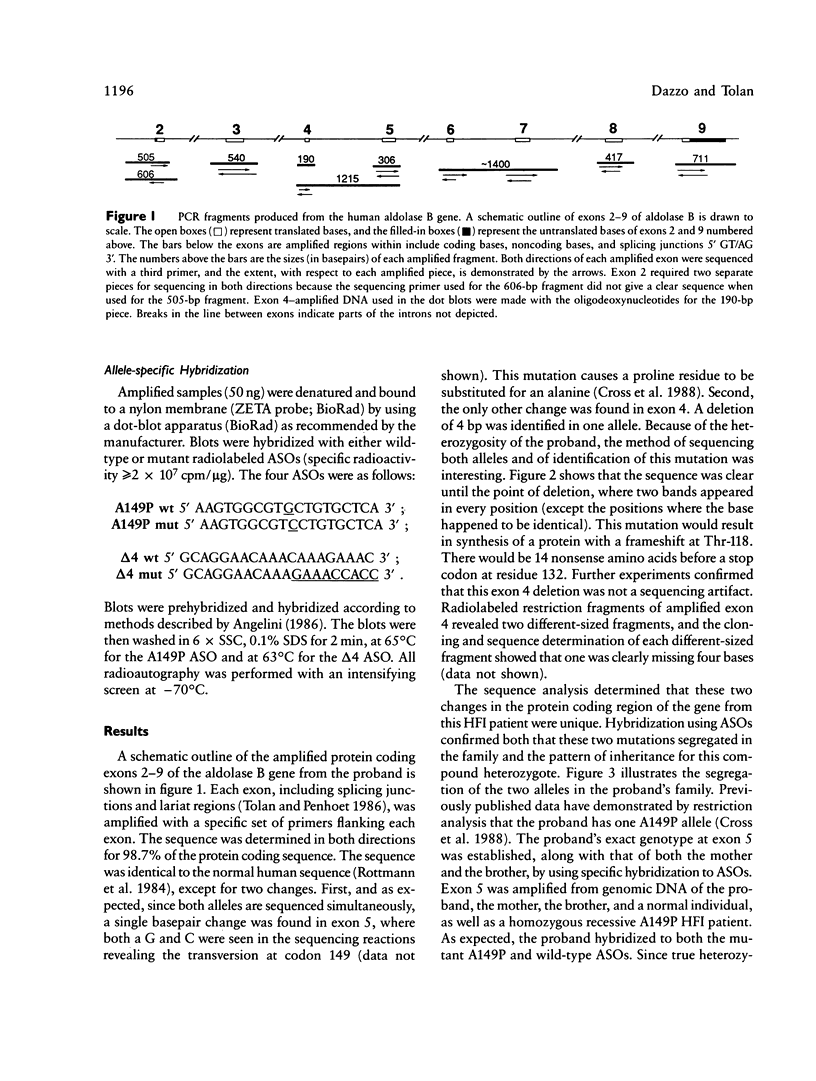
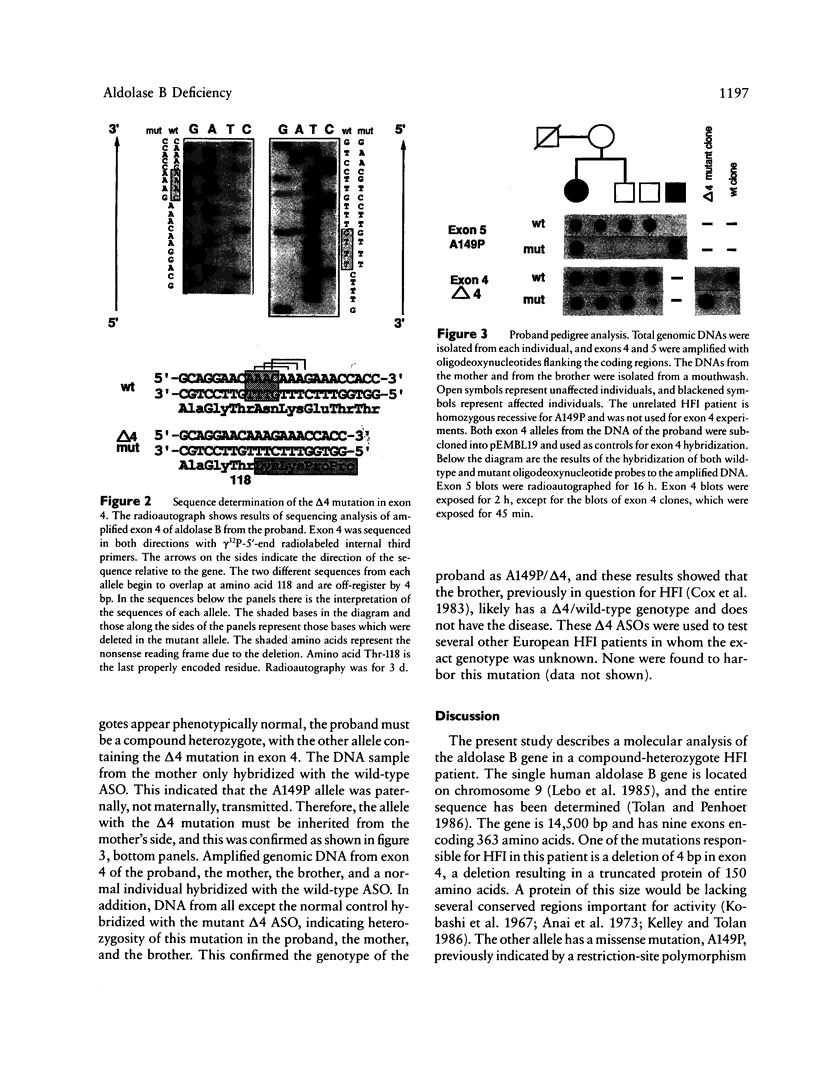
Hereditary fructose intolerance (HFI) is an inborn error of metabolism, inherited as an autosomal recessive disorder and caused by a decrease in the activity of fructose-1-phosphate aldolase (aldolase B) in affected individuals. Investigation of the molecular basis of HFI is reported here by the identification of two molecular lesions in the aldolase B gene of the HFI individual. Using polymerase chain reaction to specifically amplify exons at this locus and T7 polymerase for the sequence determination of these double-stranded fragments, we show the mutational heterogeneity of the proband. One allele, previously indicated by restriction analysis, was confirmed as A149P (Ala 149 to Pro in exon 5). The other allele was identified as a 4-bp deletion found in exon 4, a deletion which causes a frameshift at codon 118, resulting in a truncated protein of 132 amino acids. Segregation of these mutant alleles in the proband's family was shown by using allele-specific oligodeoxynucleotides to probe blots of amplified DNA. The techniques employed here represent a rapid and efficient method for detection of other mutations in families with this disease. In addition, the ability to detect mutant alleles by allele-specific hybridization offers a new method for definitive diagnosis, a method which avoids a fructose loading or liver-biopsy examination.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anai M., Lai C. Y., Horecker B. L. The pyridoxal phosphate-binding site of rabbit muscle aldolase. Arch Biochem Biophys. 1973 Jun;156(2):712–719. doi: 10.1016/0003-9861(73)90324-x. [DOI] [PubMed] [Google Scholar]
- Angelini G., de Preval C., Gorski J., Mach B. High-resolution analysis of the human HLA-DR polymorphism by hybridization with sequence-specific oligonucleotide probes. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4489–4493. doi: 10.1073/pnas.83.12.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMBERS R. A., PRATT R. T. Idiosyncrasy to fructose. Lancet. 1956 Aug 18;271(6938):340–340. doi: 10.1016/s0140-6736(56)92196-1. [DOI] [PubMed] [Google Scholar]
- Cox T. M., O'Donnell M. W., Camilleri M. Allelic heterogeneity in adult hereditary fructose intolerance. Detection of structural mutations in the aldolase B molecule. Mol Biol Med. 1983 Nov;1(4):393–400. [PubMed] [Google Scholar]
- Cross N. C., Tolan D. R., Cox T. M. Catalytic deficiency of human aldolase B in hereditary fructose intolerance caused by a common missense mutation. Cell. 1988 Jun 17;53(6):881–885. doi: 10.1016/s0092-8674(88)90349-2. [DOI] [PubMed] [Google Scholar]
- Cross N. C., de Franchis R., Sebastio G., Dazzo C., Tolan D. R., Gregori C., Odievre M., Vidailhet M., Romano V., Mascali G. Molecular analysis of aldolase B genes in hereditary fructose intolerance. Lancet. 1990 Feb 10;335(8685):306–309. doi: 10.1016/0140-6736(90)90603-3. [DOI] [PubMed] [Google Scholar]
- Kelley P. M., Tolan D. R. The complete amino Acid sequence for the anaerobically induced aldolase from maize derived from cDNA clones. Plant Physiol. 1986 Dec;82(4):1076–1080. doi: 10.1104/pp.82.4.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobashi K., Horecker B. L. Reversible inactivation of rabbit muscle aldolase by ophenanthroline. Arch Biochem Biophys. 1967 Jul;121(1):178–186. doi: 10.1016/0003-9861(67)90022-7. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Frameshift mutagenesis by eucaryotic DNA polymerases in vitro. J Biol Chem. 1986 Oct 15;261(29):13581–13587. [PubMed] [Google Scholar]
- Lameire N., Mussche M., Baele G., Kint J., Ringoir S. Hereditary fructose intolerance: a difficult diagnosis in the adult. Am J Med. 1978 Sep;65(3):416–423. doi: 10.1016/0002-9343(78)90767-2. [DOI] [PubMed] [Google Scholar]
- Lebo R. V., Tolan D. R., Bruce B. D., Cheung M. C., Kan Y. W. Spot-blot analysis of sorted chromosomes assigns a fructose intolerance disease locus to chromosome 9. Cytometry. 1985 Sep;6(5):478–483. doi: 10.1002/cyto.990060513. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Alter B. P., Altay C., Mahoney M. J., Lazarus H., Hobbins J. C., Nathan D. G. Application of endonuclease mapping to the analysis and prenatal diagnosis of thalassemias caused by globin-gene deletion. N Engl J Med. 1978 Jul 27;299(4):166–172. doi: 10.1056/NEJM197807272990403. [DOI] [PubMed] [Google Scholar]
- Rottmann W. H., Tolan D. R., Penhoet E. E. Complete amino acid sequence for human aldolase B derived from cDNA and genomic clones. Proc Natl Acad Sci U S A. 1984 May;81(9):2738–2742. doi: 10.1073/pnas.81.9.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]