Abstract
XR-1 is a Chinese hamster ovary (CHO) cell mutant which is unusually sensitive to killing by gamma rays in the G1 portion of the cell cycle but has nearly normal resistance to gamma-ray damage in late S phase. The cell-cycle sensitivity correlates with the mutant's inability to repair DNA double-strand breaks (DSBs) produced by ionizing radiation and restriction enzymes. We have previously shown in somatic cell hybrids of XR-1 cells and human fibroblasts that the XR-1 mutation is a recessive mutation. In this study, using somatic cell hybrids formed between XR-1 and human fibroblasts, we map the human complementing gene to chromosome 5 by chromosome-segregation analysis. This gene biochemically restores the hamster defect to wild-type levels of gamma-ray and bleomycin resistance as well as restoring its proficiency to repair DNA DSBs, suggesting that a single gene is responsible for the XR-1 phenotype. We have tentatively assigned the name XRCC4 (X-ray-complementing Chinese hamster gene 4) to this human gene until its biochemical function in repair is discovered.
Full text
PDF


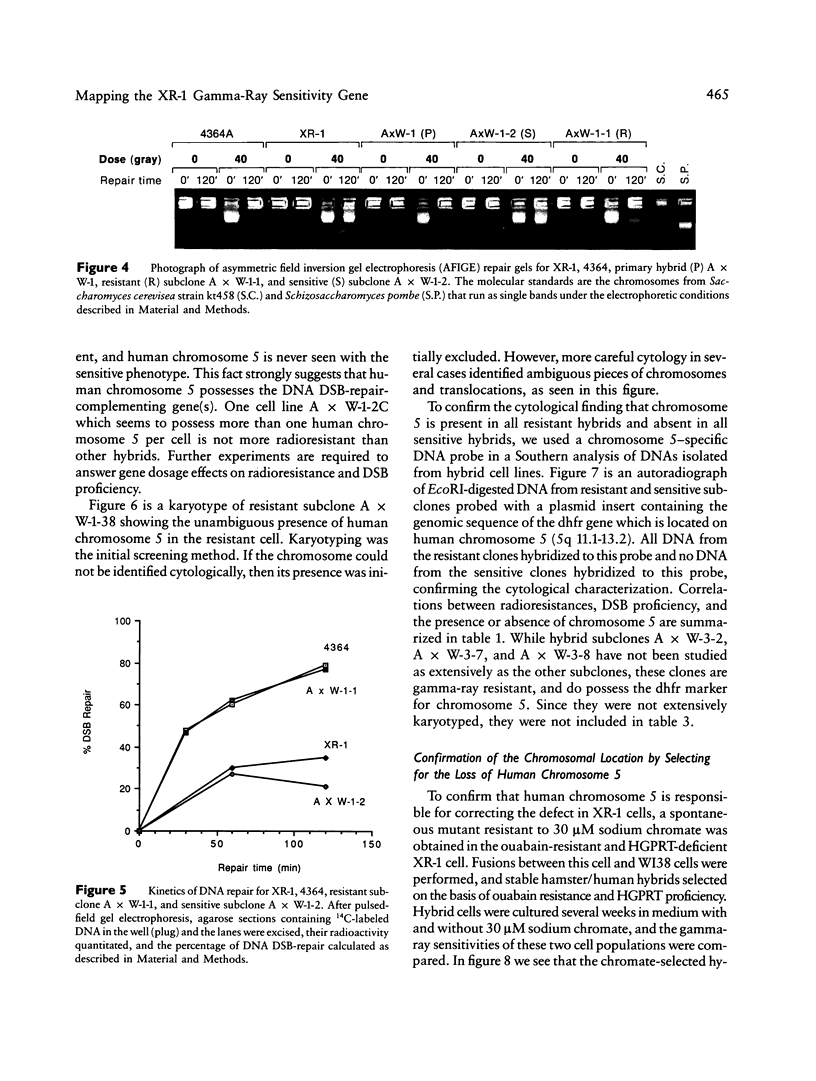
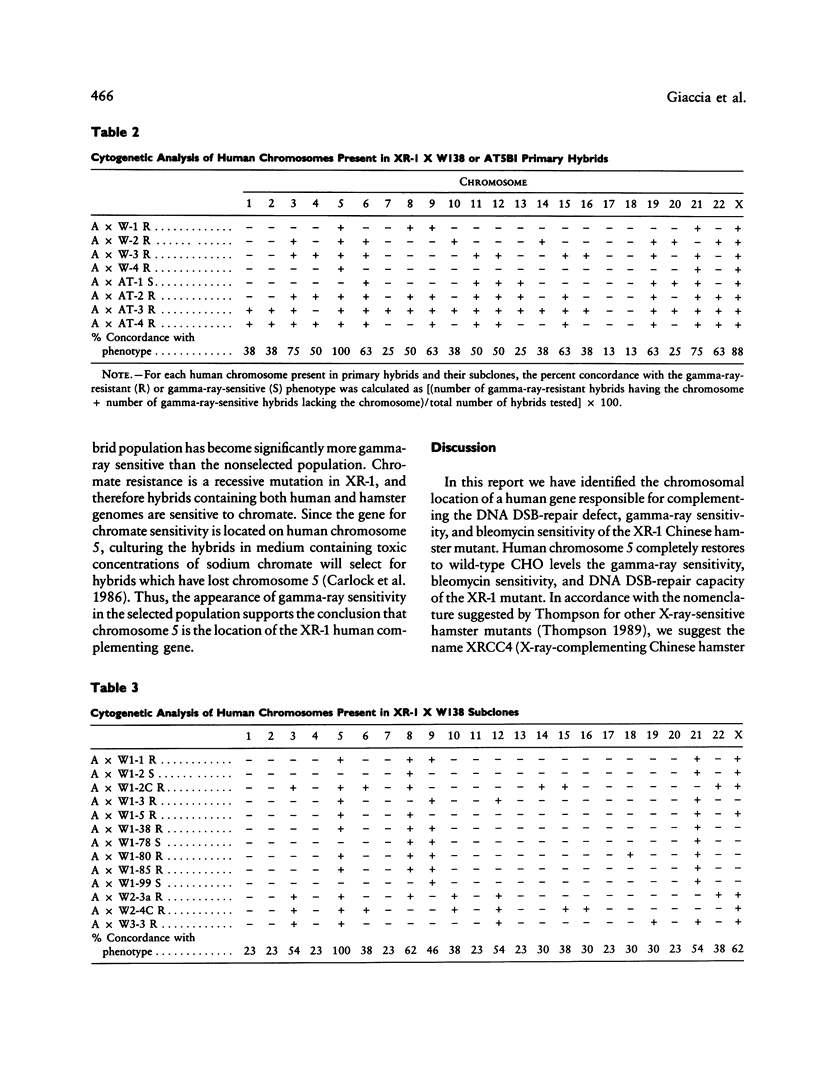
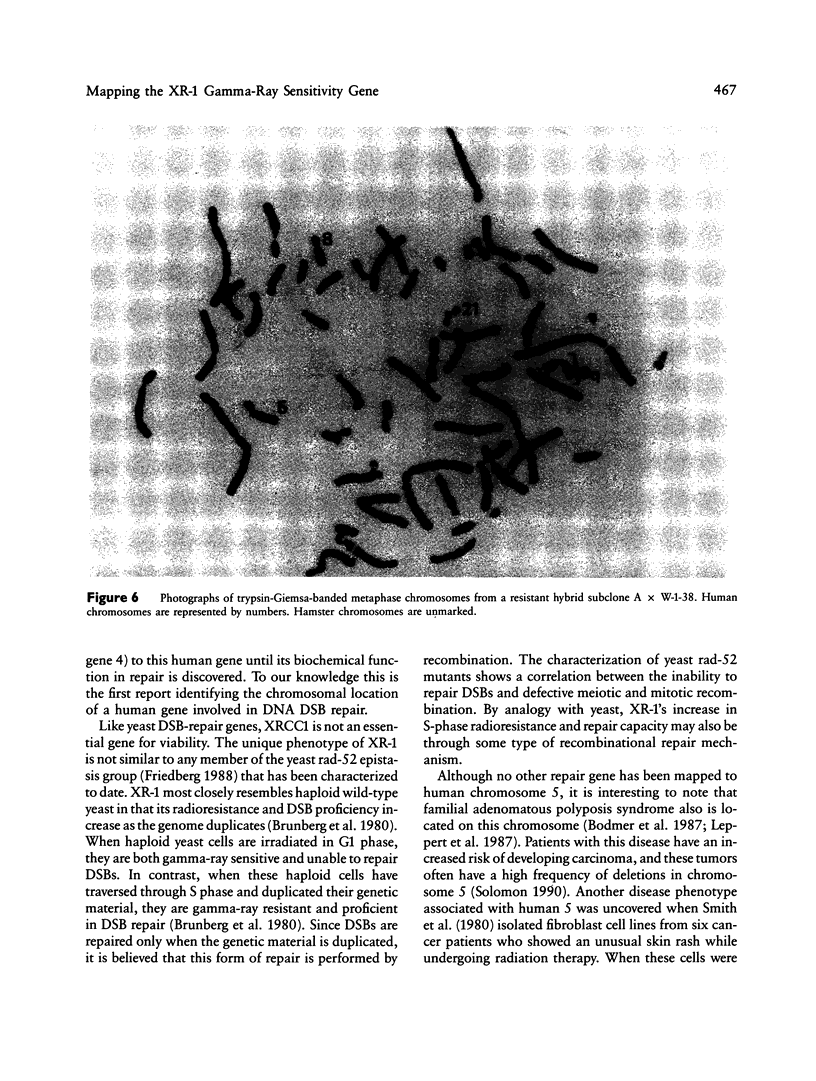
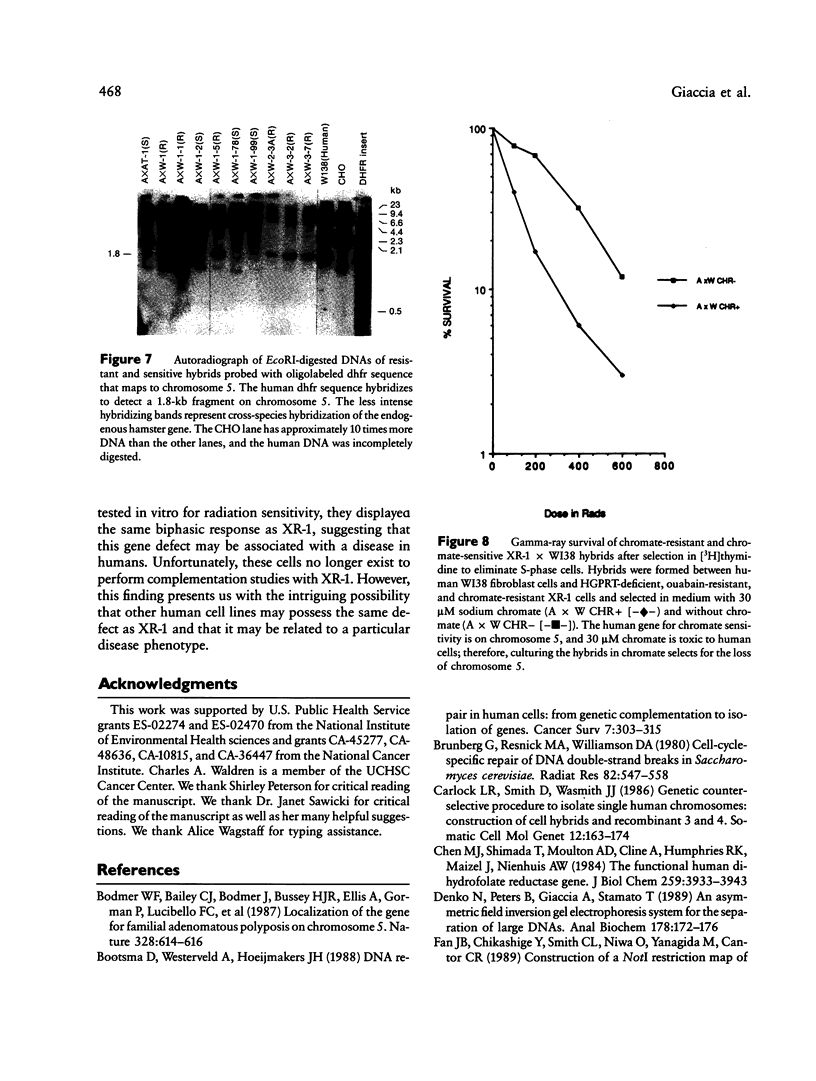

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodmer W. F., Bailey C. J., Bodmer J., Bussey H. J., Ellis A., Gorman P., Lucibello F. C., Murday V. A., Rider S. H., Scambler P. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature. 1987 Aug 13;328(6131):614–616. doi: 10.1038/328614a0. [DOI] [PubMed] [Google Scholar]
- Brunborg G., Resnick M. A., Williamson D. H. Cell-cycle-specific repair of DNA double strand breaks in Saccharomyces cerevisiae. Radiat Res. 1980 Jun;82(3):547–558. [PubMed] [Google Scholar]
- Carlock L. R., Smith D., Wasmuth J. J. Genetic counterselective procedure to isolate interspecific cell hybrids containing single human chromosomes: construction of cell hybrids and recombinant DNA libraries specific for human chromosomes 3 and 4. Somat Cell Mol Genet. 1986 Mar;12(2):163–174. doi: 10.1007/BF01560663. [DOI] [PubMed] [Google Scholar]
- Chen M. J., Shimada T., Moulton A. D., Cline A., Humphries R. K., Maizel J., Nienhuis A. W. The functional human dihydrofolate reductase gene. J Biol Chem. 1984 Mar 25;259(6):3933–3943. [PubMed] [Google Scholar]
- Denko N., Giaccia A., Peters B., Stamato T. D. An asymmetric field inversion gel electrophoresis method for the separation of large DNA molecules. Anal Biochem. 1989 Apr;178(1):172–176. doi: 10.1016/0003-2697(89)90375-8. [DOI] [PubMed] [Google Scholar]
- Fan J. B., Chikashige Y., Smith C. L., Niwa O., Yanagida M., Cantor C. R. Construction of a Not I restriction map of the fission yeast Schizosaccharomyces pombe genome. Nucleic Acids Res. 1989 Apr 11;17(7):2801–2818. doi: 10.1093/nar/17.7.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C. Deoxyribonucleic acid repair in the yeast Saccharomyces cerevisiae. Microbiol Rev. 1988 Mar;52(1):70–102. doi: 10.1128/mr.52.1.70-102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend K. K., Chen S., Ruddle F. H. Differential staining of interspecific chromosomes in somatic cell hybrids by alkaline Giemsa stain. Somatic Cell Genet. 1976 Mar;2(2):183–188. doi: 10.1007/BF01542631. [DOI] [PubMed] [Google Scholar]
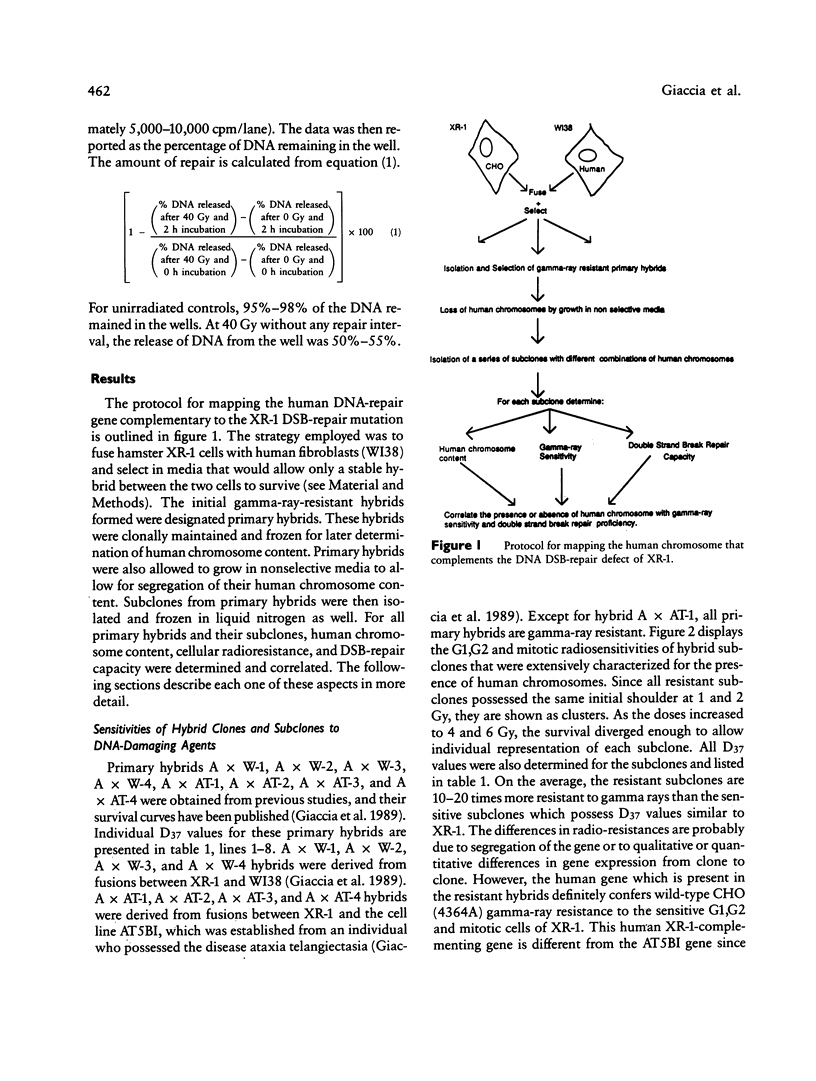
- Giaccia A. J., Richardson E., Denko N., Stamato T. D. Genetic analysis of XR-1 mutation in hamster and human hybrids. Somat Cell Mol Genet. 1989 Jan;15(1):71–77. doi: 10.1007/BF01534671. [DOI] [PubMed] [Google Scholar]
- Giaccia A., Weinstein R., Hu J., Stamato T. D. Cell cycle-dependent repair of double-strand DNA breaks in a gamma-ray-sensitive Chinese hamster cell. Somat Cell Mol Genet. 1985 Sep;11(5):485–491. doi: 10.1007/BF01534842. [DOI] [PubMed] [Google Scholar]
- Hickson I. D., Harris A. L. Mammalian DNA repair--use of mutants hypersensitive to cytotoxic agents. Trends Genet. 1988 Apr;4(4):101–106. doi: 10.1016/0168-9525(88)90097-2. [DOI] [PubMed] [Google Scholar]
- Jeggo P. A. Genetic analysis of X-ray-sensitive mutants of the CHO cell line. Mutat Res. 1985 Nov;146(3):265–270. doi: 10.1016/0167-8817(85)90067-7. [DOI] [PubMed] [Google Scholar]
- Jeggo P. A., Holliday R. Azacytidine-induced reactivation of a DNA repair gene in Chinese hamster ovary cells. Mol Cell Biol. 1986 Aug;6(8):2944–2949. doi: 10.1128/mcb.6.8.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeggo P. A., Kemp L. M. X-ray-sensitive mutants of Chinese hamster ovary cell line. Isolation and cross-sensitivity to other DNA-damaging agents. Mutat Res. 1983 Dec;112(6):313–327. doi: 10.1016/0167-8817(83)90026-3. [DOI] [PubMed] [Google Scholar]
- Jones N. J., Cox R., Thacker J. Six complementation groups for ionising-radiation sensitivity in Chinese hamster cells. Mutat Res. 1988 Mar;193(2):139–144. doi: 10.1016/0167-8817(88)90044-2. [DOI] [PubMed] [Google Scholar]
- Kemp L. M., Sedgwick S. G., Jeggo P. A. X-ray sensitive mutants of Chinese hamster ovary cells defective in double-strand break rejoining. Mutat Res. 1984 Nov-Dec;132(5-6):189–196. doi: 10.1016/0167-8817(84)90037-3. [DOI] [PubMed] [Google Scholar]
- Leppert M., Dobbs M., Scambler P., O'Connell P., Nakamura Y., Stauffer D., Woodward S., Burt R., Hughes J., Gardner E. The gene for familial polyposis coli maps to the long arm of chromosome 5. Science. 1987 Dec 4;238(4832):1411–1413. doi: 10.1126/science.3479843. [DOI] [PubMed] [Google Scholar]
- Nagasawa H., Cox A. B., Lett J. T. The radiation responses of synchronous L5178Y S/S cells and their significance for radiobiological theory. Proc R Soc Lond B Biol Sci. 1980 Dec 31;211(1182):25–49. doi: 10.1098/rspb.1980.0156. [DOI] [PubMed] [Google Scholar]
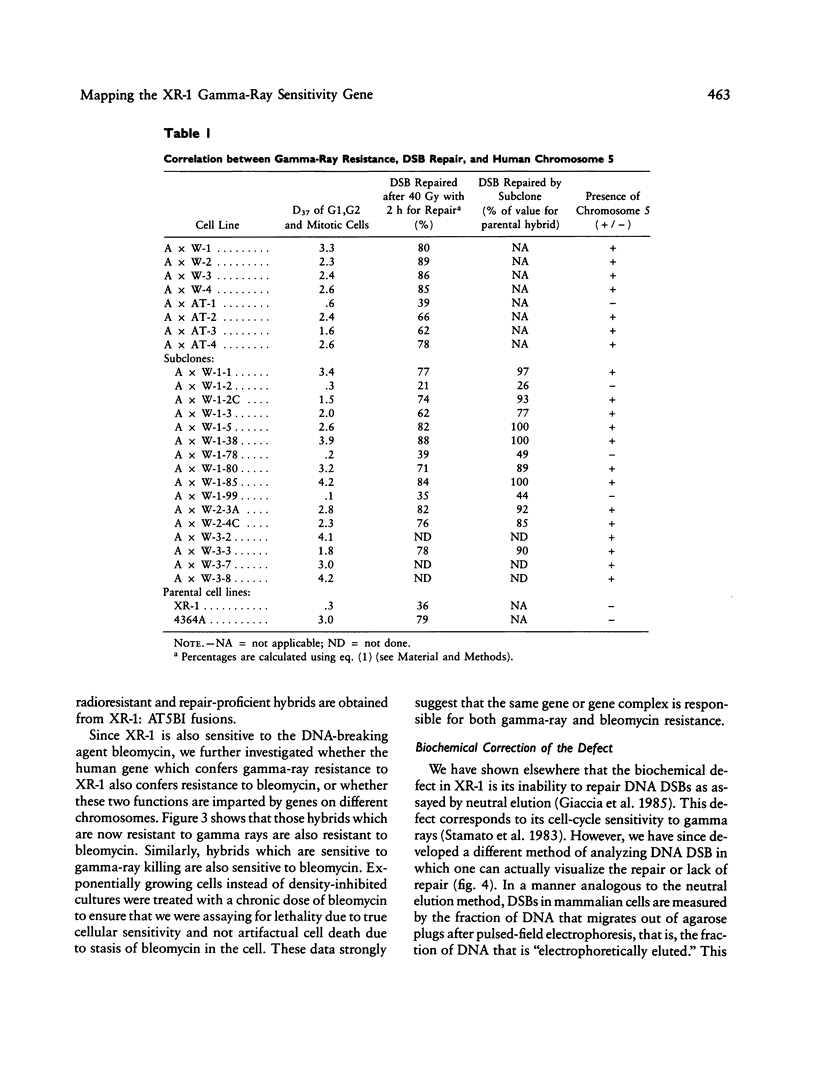
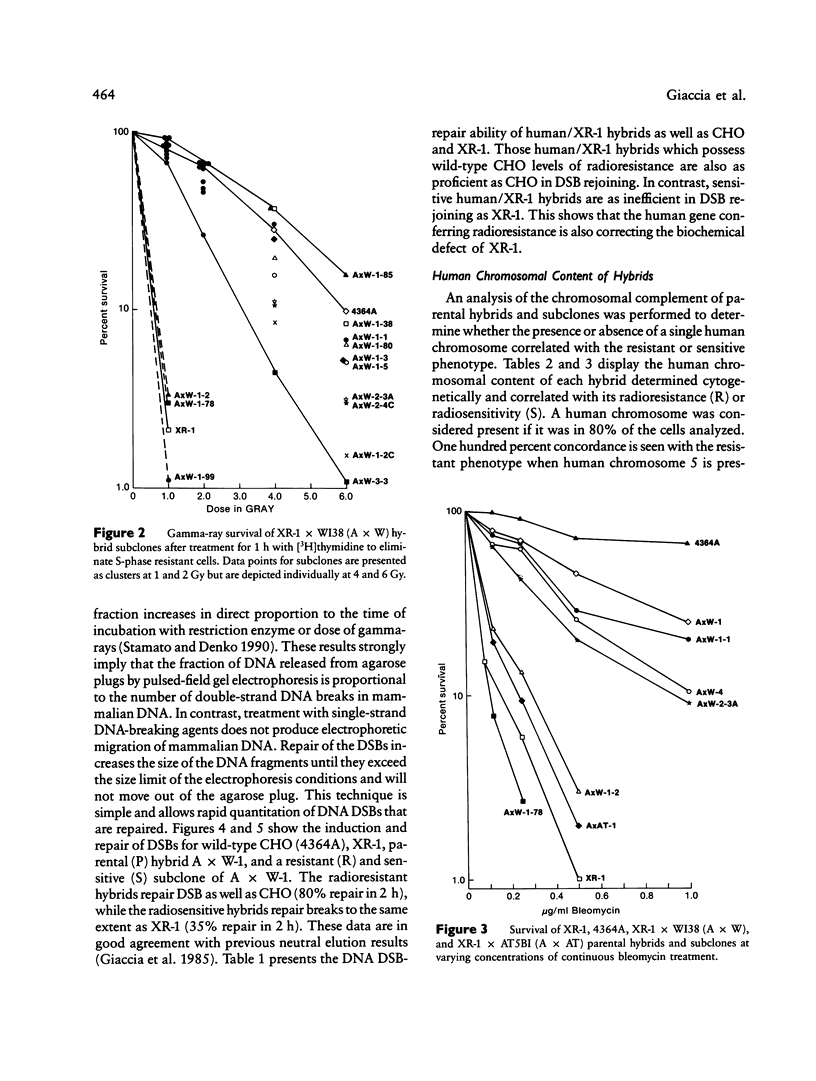
- Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971 Oct 30;2(7731):971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Siciliano M. J., Carrano A. V., Thompson L. H. Assignment of a human DNA-repair gene associated with sister-chromatid exchange to chromosome 19. Mutat Res. 1986 Aug;174(4):303–308. doi: 10.1016/0165-7992(86)90051-5. [DOI] [PubMed] [Google Scholar]
- Smith K. C., Hahn G. M., Hoppe R. T., Earle J. D. Radiosensitivity in vitro of human fibroblasts derived from patients with a severe skin reaction to radiation therapy. Int J Radiat Oncol Biol Phys. 1980 Nov;6(11):1573–1575. doi: 10.1016/0360-3016(80)90017-6. [DOI] [PubMed] [Google Scholar]
- Solomon E. Molecular genetics. Colorectal cancer genes. Nature. 1990 Feb 1;343(6257):412–414. doi: 10.1038/343412a0. [DOI] [PubMed] [Google Scholar]
- Stamato T. D., Denko N. Asymmetric field inversion gel electrophoresis: a new method for detecting DNA double-strand breaks in mammalian cells. Radiat Res. 1990 Feb;121(2):196–205. [PubMed] [Google Scholar]
- Stamato T. D., Dipatri A., Giaccia A. Cell-cycle-dependent repair of potentially lethal damage in the XR-1 gamma-ray-sensitive Chinese hamster ovary cell. Radiat Res. 1988 Aug;115(2):325–333. [PubMed] [Google Scholar]
- Stamato T. D., Peters B., Patil P., Denko N., Weinstein R., Giaccia A. Isolation and characterization of bleomycin-sensitive Chinese hamster ovary cells. Cancer Res. 1987 Mar 15;47(6):1588–1592. [PubMed] [Google Scholar]
- Stamato T. D., Weinstein R., Giaccia A., Mackenzie L. Isolation of cell cycle-dependent gamma ray-sensitive Chinese hamster ovary cell. Somatic Cell Genet. 1983 Mar;9(2):165–173. doi: 10.1007/BF01543175. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Dillehay L. E., Carrano A. V., Mazrimas J. A., Mooney C. L., Minkler J. L. A CHO-cell strain having hypersensitivity to mutagens, a defect in DNA strand-break repair, and an extraordinary baseline frequency of sister-chromatid exchange. Mutat Res. 1982 Aug;95(2-3):427–440. doi: 10.1016/0027-5107(82)90276-7. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Salazar E. P., Brookman K. W., Collins C. C., Stewart S. A., Busch D. B., Weber C. A. Recent progress with the DNA repair mutants of Chinese hamster ovary cells. J Cell Sci Suppl. 1987;6:97–110. doi: 10.1242/jcs.1984.supplement_6.6. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Shiomi T., Salazar E. P., Stewart S. A. An eighth complementation group of rodent cells hypersensitive to ultraviolet radiation. Somat Cell Mol Genet. 1988 Nov;14(6):605–612. doi: 10.1007/BF01535314. [DOI] [PubMed] [Google Scholar]
- Thompson L. H. Somatic cell genetics approach to dissecting mammalian DNA repair. Environ Mol Mutagen. 1989;14(4):264–281. doi: 10.1002/em.2850140409. [DOI] [PubMed] [Google Scholar]
- Weber C. A., Salazar E. P., Stewart S. A., Thompson L. H. Molecular cloning and biological characterization of a human gene, ERCC2, that corrects the nucleotide excision repair defect in CHO UV5 cells. Mol Cell Biol. 1988 Mar;8(3):1137–1146. doi: 10.1128/mcb.8.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duin M., de Wit J., Odijk H., Westerveld A., Yasui A., Koken M. H., Hoeijmakers J. H., Bootsma D. Molecular characterization of the human excision repair gene ERCC-1: cDNA cloning and amino acid homology with the yeast DNA repair gene RAD10. Cell. 1986 Mar 28;44(6):913–923. doi: 10.1016/0092-8674(86)90014-0. [DOI] [PubMed] [Google Scholar]