Abstract
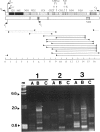
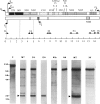
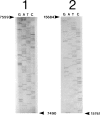
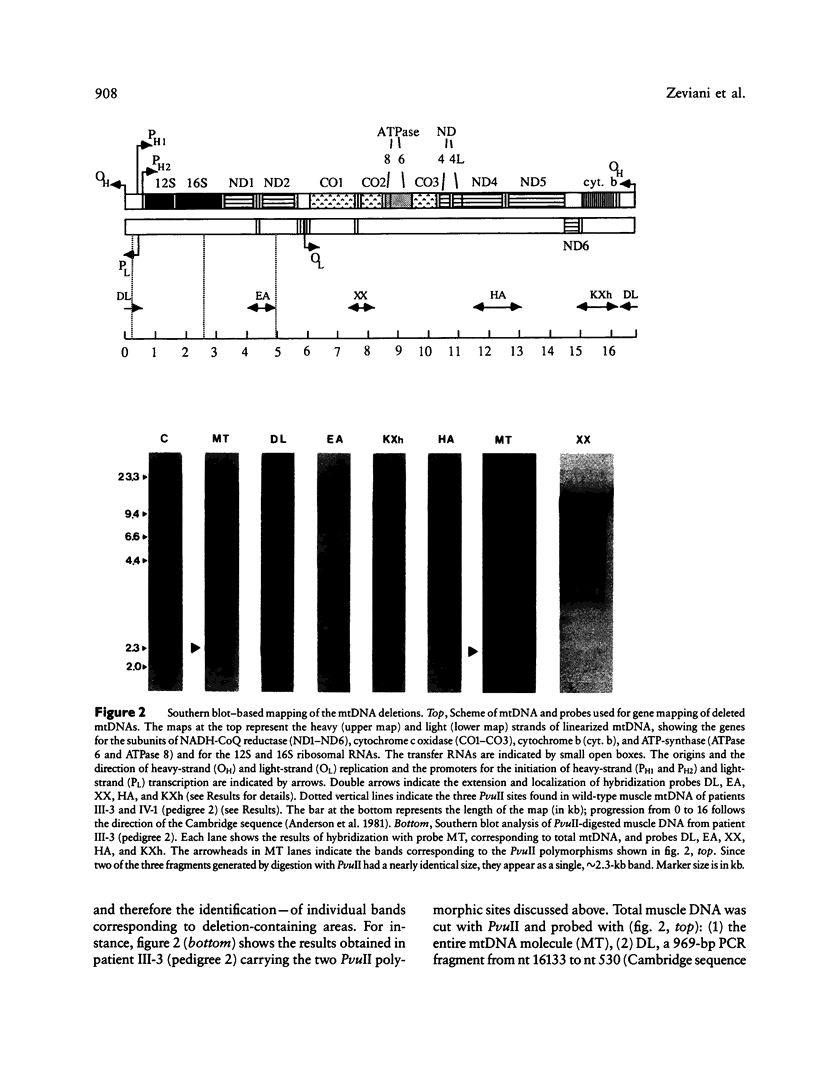
We studied several affected and one nonaffected individuals belonging to three unrelated pedigrees. The pathological trait was an autosomal dominant mitochondrial myopathy due to large-scale multiple deletions of the mitochondrial genome. Clinically, symptomatic patients had progressive external ophthalmoplegia, muscle weakness and wasting, sensorineural hypoacusia, and, in some cases, vestibular areflexia and tremor. The muscle biopsies of all patients examined showed ragged-red fibers, neurogenic changes, and a partially decreased histochemical reaction to cytochrome c oxidase. Multiple mtDNA heteroplasmy was detected in the patients by both Southern blot analysis and PCR amplification, whereas the unaffected individual had the normal homoplasmic hybridization pattern. These findings confirm and add further details to the existence of a new human disease--defined clinically as a mitochondrial myopathy, genetically as a Mendelian autosomal dominant trait, and molecularly by the accumulation of multiple, large-scale deletions of the mitochondrial genome--that is due to impaired nuclear control during mtDNA replication.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Cooper J. M., Schapira A. H., Toscano A., Clark J. B., Morgan-Hughes J. A. Mitochondrial myopathies: clinical and biochemical features of 30 patients with major deletions of muscle mitochondrial DNA. Ann Neurol. 1989 Dec;26(6):699–708. doi: 10.1002/ana.410260603. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Morgan-Hughes J. A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988 Feb 25;331(6158):717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- Husain M., Steenkamp D. J. Electron transfer flavoprotein from pig liver mitochondria. A simple purification and re-evaluation of some of the molecular properties. Biochem J. 1983 Feb 1;209(2):541–545. doi: 10.1042/bj2090541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Newbold J. E., Potter S. S., Edgell M. H. Maternal inheritance of mammalian mitochondrial DNA. Nature. 1974 Oct 11;251(5475):536–538. doi: 10.1038/251536a0. [DOI] [PubMed] [Google Scholar]
- Johns D. R., Drachman D. B., Hurko O. Identical mitochondrial DNA deletion in blood and muscle. Lancet. 1989 Feb 18;1(8634):393–394. doi: 10.1016/s0140-6736(89)91779-0. [DOI] [PubMed] [Google Scholar]
- Johns D. R., Rutledge S. L., Stine O. C., Hurko O. Directly repeated sequences associated with pathogenic mitochondrial DNA deletions. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8059–8062. doi: 10.1073/pnas.86.20.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombes A., Bonilla E., Dimauro S. Mitochondrial encephalomyopathies. Rev Neurol (Paris) 1989;145(10):671–689. [PubMed] [Google Scholar]
- Mignotte B., Barat M., Mounolou J. C. Characterization of a mitochondrial protein binding to single-stranded DNA. Nucleic Acids Res. 1985 Mar 11;13(5):1703–1716. doi: 10.1093/nar/13.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita S., Rizzuto R., Moraes C. T., Shanske S., Arnaudo E., Fabrizi G. M., Koga Y., DiMauro S., Schon E. A. Recombination via flanking direct repeats is a major cause of large-scale deletions of human mitochondrial DNA. Nucleic Acids Res. 1990 Feb 11;18(3):561–567. doi: 10.1093/nar/18.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes C. T., DiMauro S., Zeviani M., Lombes A., Shanske S., Miranda A. F., Nakase H., Bonilla E., Werneck L. C., Servidei S. Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns-Sayre syndrome. N Engl J Med. 1989 May 18;320(20):1293–1299. doi: 10.1056/NEJM198905183202001. [DOI] [PubMed] [Google Scholar]
- Palva T. K., Palva E. T. Rapid isolation of animal mitochondrial DNA by alkaline extraction. FEBS Lett. 1985 Nov 18;192(2):267–270. doi: 10.1016/0014-5793(85)80122-8. [DOI] [PubMed] [Google Scholar]
- Rand D. M., Harrison R. G. Molecular population genetics of mtDNA size variation in crickets. Genetics. 1989 Mar;121(3):551–569. doi: 10.1093/genetics/121.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon E. A., Rizzuto R., Moraes C. T., Nakase H., Zeviani M., DiMauro S. A direct repeat is a hotspot for large-scale deletion of human mitochondrial DNA. Science. 1989 Apr 21;244(4902):346–349. doi: 10.1126/science.2711184. [DOI] [PubMed] [Google Scholar]
- Shanske S., Moraes C. T., Lombes A., Miranda A. F., Bonilla E., Lewis P., Whelan M. A., Ellsworth C. A., DiMauro S. Widespread tissue distribution of mitochondrial DNA deletions in Kearns-Sayre syndrome. Neurology. 1990 Jan;40(1):24–28. doi: 10.1212/wnl.40.1.24. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Voljavec A. S., Soueidan S. A., Costigan D. A., Wallace D. C. Spontaneous Kearns-Sayre/chronic external ophthalmoplegia plus syndrome associated with a mitochondrial DNA deletion: a slip-replication model and metabolic therapy. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7952–7956. doi: 10.1073/pnas.86.20.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Van Tuyle G. C., Pavco P. A. Characterization of a rat liver mitochondrial DNA-protein complex. Replicative intermediates are protected against branch migrational loss. J Biol Chem. 1981 Dec 25;256(24):12772–12779. [PubMed] [Google Scholar]
- Wallace D. C. Structure and evolution of organelle genomes. Microbiol Rev. 1982 Jun;46(2):208–240. doi: 10.1128/mr.46.2.208-240.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship P. R. An improved method for directly sequencing PCR amplified material using dimethyl sulphoxide. Nucleic Acids Res. 1989 Feb 11;17(3):1266–1266. doi: 10.1093/nar/17.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzaki M., Ohkoshi N., Kanazawa I., Kagawa Y., Ohta S. Multiple deletions in mitochondrial DNA at direct repeats of non-D-loop regions in cases of familial mitochondrial myopathy. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1352–1357. doi: 10.1016/0006-291x(89)91818-4. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Gellera C., Pannacci M., Uziel G., Prelle A., Servidei S., DiDonato S. Tissue distribution and transmission of mitochondrial DNA deletions in mitochondrial myopathies. Ann Neurol. 1990 Jul;28(1):94–97. doi: 10.1002/ana.410280118. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Moraes C. T., DiMauro S., Nakase H., Bonilla E., Schon E. A., Rowland L. P. Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology. 1988 Sep;38(9):1339–1346. doi: 10.1212/wnl.38.9.1339. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Servidei S., Gellera C., Bertini E., DiMauro S., DiDonato S. An autosomal dominant disorder with multiple deletions of mitochondrial DNA starting at the D-loop region. Nature. 1989 May 25;339(6222):309–311. doi: 10.1038/339309a0. [DOI] [PubMed] [Google Scholar]