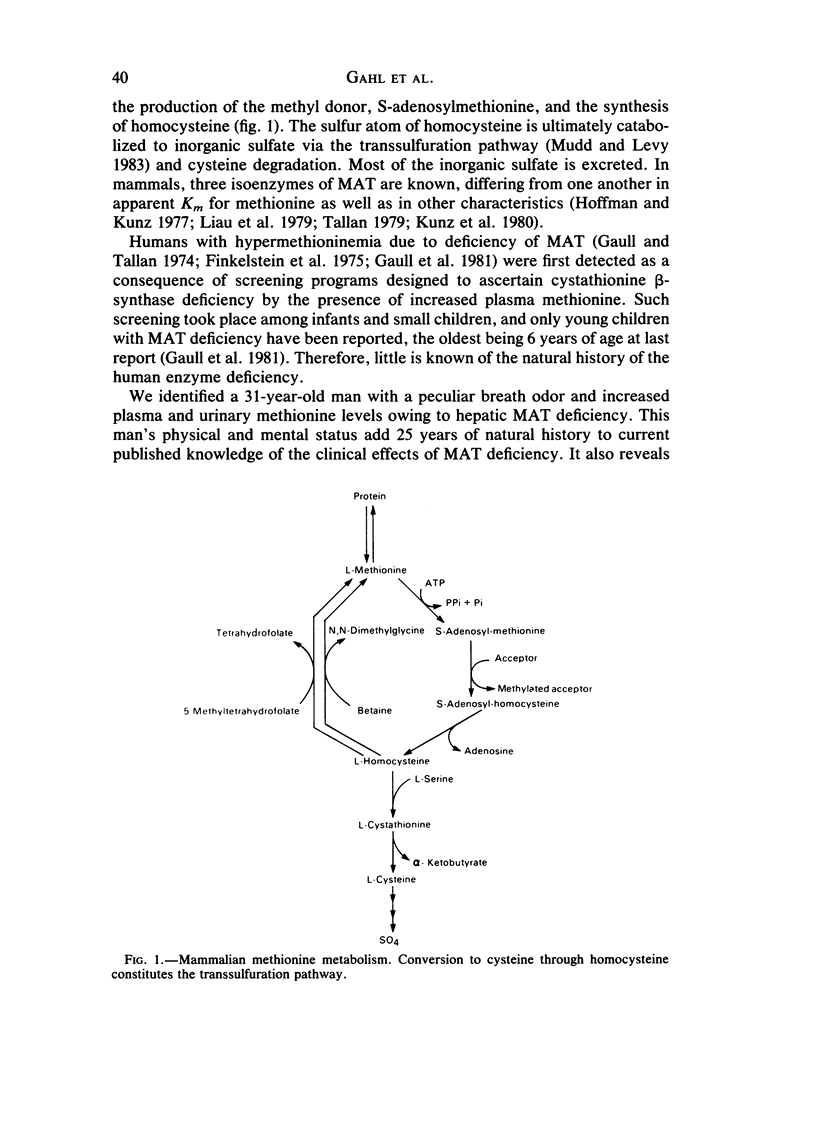
Abstract
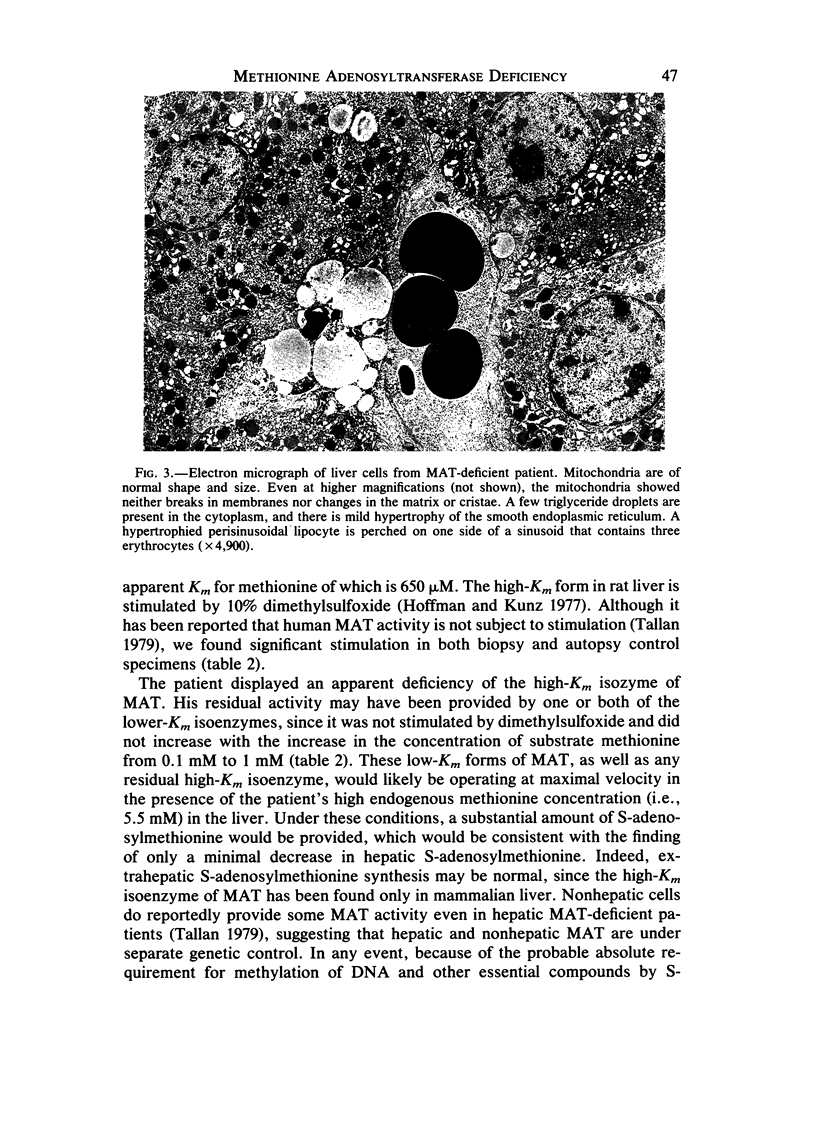
A 31-year-old man with hepatic methionine adenosyltransferase (MAT) deficiency was evaluated for an odd odor to his breath. He had no other symptoms. Plasma methionine was 716 microM (normal, 15-40 microM), and plasma methionine-oxidation products were 460 microM (normal, 0). Hepatic MAT activity was 28% of normal. Unlike the control human enzyme, the patient's residual MAT activity was not stimulated by 10% dimethylsulfoxide and the velocity was not increased by high substrate concentration; at 1.0 mM methionine, the patient's MAT activity was only 7% of normal. These biochemical findings are consistent with a deficiency of the high-Km isoenzyme of MAT. Despite this enzyme deficiency, liver histology and clinical tests of hepatic and other organ function were normal. The patient, who is 25 years older than the oldest reported individual with MAT deficiency, provides evidence that partial MAT deficiency is a benign disorder and that chronic hypermethioninemia (less than 1 mM) is not by itself detrimental to health.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldessarini R. J. Biological transmethylation involving S-adenosylmethionine: development of assay methods and implications for neuropsychiatry. Int Rev Neurobiol. 1975;18:41–67. doi: 10.1016/s0074-7742(08)60033-1. [DOI] [PubMed] [Google Scholar]
- Bernardini I., Rizzo W. B., Dalakas M., Bernar J., Gahl W. A. Plasma and muscle free carnitine deficiency due to renal Fanconi syndrome. J Clin Invest. 1985 Apr;75(4):1124–1130. doi: 10.1172/JCI111806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein J. D., Kyle W. E., Martin J. J. Abnormal methionine adenosyltransferase in hypermethioninemia. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1491–1497. doi: 10.1016/0006-291x(75)90527-6. [DOI] [PubMed] [Google Scholar]
- Finkelstein J. D., Mudd S. H. Trans-sulfuration in mammals. The methionine-sparing effect of cystine. J Biol Chem. 1967 Mar 10;242(5):873–880. [PubMed] [Google Scholar]
- Gaull G. E., Bender A. N., Vulovic D., Tallan H. H., Schaffner F. Methioninemia and myopathy: a new disorder. Ann Neurol. 1981 May;9(5):423–432. doi: 10.1002/ana.410090503. [DOI] [PubMed] [Google Scholar]
- Gaull G. E., Tallan H. H., Lonsdale D., Przyrembel H., Schaffner F., von Bassewitz D. B. Hypermethioninemia associated with methionine adenosyltransferase deficiency: clinical, morphologic, and biochemical observations on four patients. J Pediatr. 1981 May;98(5):734–741. doi: 10.1016/s0022-3476(81)80833-5. [DOI] [PubMed] [Google Scholar]
- Gaull G. E., Tallan H. H. Methionine adenosyltransferase deficiency: new enzymatic defect associated with hypermethioninemia. Science. 1974 Oct 4;186(4158):59–60. doi: 10.1126/science.186.4158.59. [DOI] [PubMed] [Google Scholar]
- Hoffman J. L., Kunz G. L. Differential activation of rat liver methionine adenosyltransferase isozymes by dimethylsulfoxide. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1231–1236. doi: 10.1016/s0006-291x(77)80111-3. [DOI] [PubMed] [Google Scholar]
- Hoffman J. A rapid liquid chromatographic determination of S-adenosylhomocysteine in subgram amounts of tissue. Anal Biochem. 1975 Oct;68(2):522–530. doi: 10.1016/0003-2697(75)90647-8. [DOI] [PubMed] [Google Scholar]
- Kunz G. L., Hoffman J. L., Chia C. S., Stremel B. Separation of rat liver methionine adenosyltransferase isozymes by hydrophobic chromatography. Arch Biochem Biophys. 1980 Jul;202(2):565–572. doi: 10.1016/0003-9861(80)90463-4. [DOI] [PubMed] [Google Scholar]
- Levy H. L., Mudd S. H., Schulman J. D., Dreyfus P. M., Abeles R. H. A derangement in B12 metabolism associated with homocystinemia, cystathioninemia, hypomethioninemia and methylmalonic aciduria. Am J Med. 1970 Mar;48(3):390–397. doi: 10.1016/0002-9343(70)90070-7. [DOI] [PubMed] [Google Scholar]
- Liau M. C., Chang C. F., Belanger L., Grenier A. Correlation of isozyme patterns of S-adenosylmethionine synthetase with fetal stages and pathological states of the liver. Cancer Res. 1979 Jan;39(1):162–169. [PubMed] [Google Scholar]
- Mudd S. H., Ebert M. H., Scriver C. R. Labile methyl group balances in the human: the role of sarcosine. Metabolism. 1980 Aug;29(8):707–720. doi: 10.1016/0026-0495(80)90192-4. [DOI] [PubMed] [Google Scholar]
- Mudd S. H., Finkelstein J. D., Irreverre F., Laster L. Transsulfuration in mammals. Microassays and tissue distributions of three enzymes of the pathway. J Biol Chem. 1965 Nov;240(11):4382–4392. [PubMed] [Google Scholar]
- Tallan H. H. Methionine adenosyltransferase in man: evidence for multiple forms. Biochem Med. 1979 Apr;21(2):129–140. doi: 10.1016/0006-2944(79)90064-4. [DOI] [PubMed] [Google Scholar]




