Abstract
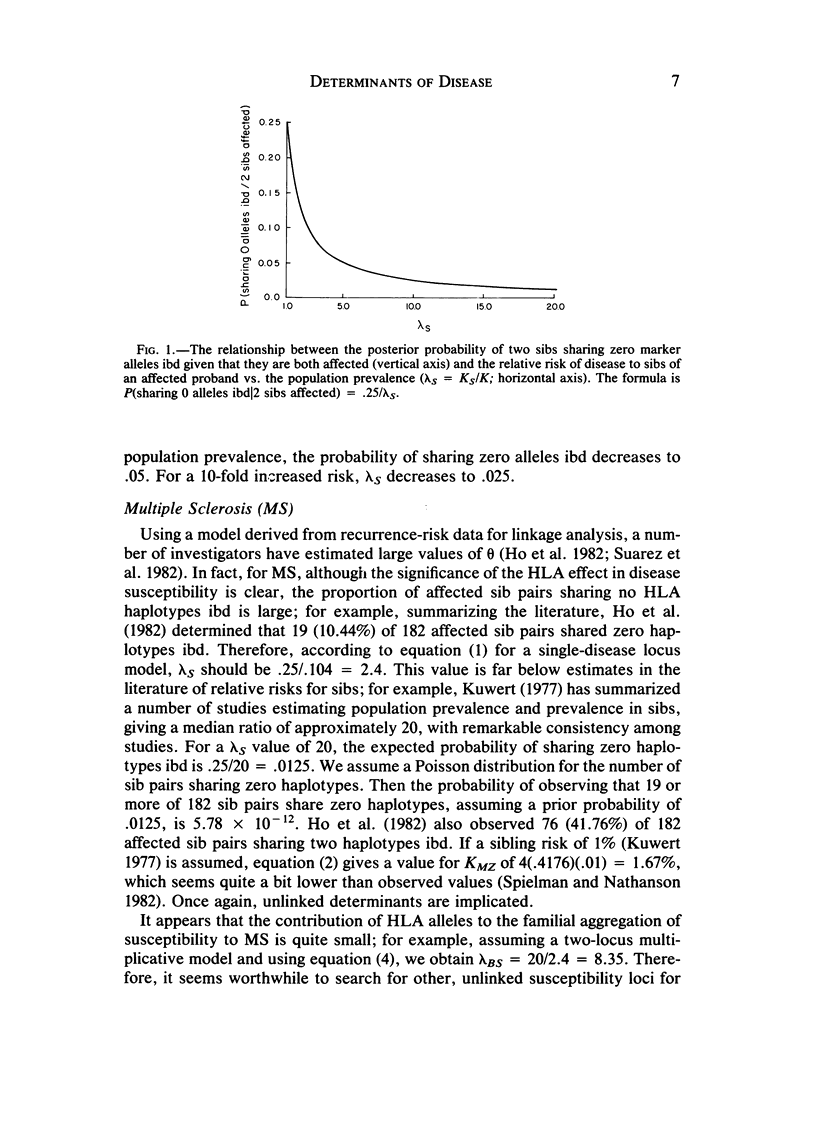
The relationship between increased risk in relatives over population prevalence (lambda R = KR/K) and probability of sharing zero marker alleles identical by descent (ibd) at a linked locus (such as HLA) by an affected relative pair is examined. For a model assuming a single disease-susceptibility locus or group of loci tightly linked to a marker locus, the relationship is remarkably simple and general. Namely, if phi R is the prior probability for the relative pair to share zero marker alleles identical by descent, then P (sharing 0 markers/both relatives are affected) is just phi R/lambda R. Alternatively, lambda AR, the increased risk over population prevalence to a relative R due to a disease locus tightly linked to marker locus A, equals the prior probability that the relative pair share zero A alleles ibd divided by the posterior probability that they share zero alleles ibd, given that they are both affected. For example, for affected sib pairs, P (sharing 0 markers/both sibs are affected) = .25/lambda S. This formula holds true for any number of alleles at the disease locus and for their frequencies, penetrances, and population prevalence. Similar formulas are derived for sharing one and two markers. Application of these formulas to several well-studied HLA-associated diseases yields the following results: For multiple sclerosis, insulin-dependent diabetes mellitus, and coeliac disease, a single-locus model of disease susceptibility is rejected, implying the existence of additional unlinked familial determinants. For all three diseases, the effect of the HLA-linked locus on familiality is minor: for multiple sclerosis, it accounts for only a 2.5-fold increased risk to sibs over the population prevalence, compared to an observed value of 20; for coeliac disease, it accounts for approximately a 5.25-fold increased risk to sibs, while the observed value is on the order of 60; for insulin-dependent diabetes mellitus, it accounts for a 3.42-fold increased risk in sibs, while the observed value is 15. In all cases, the secondary determinants must be outside the HLA region. For tuberculoid leprosy, an unlinked familial determinant is also implicated (increased risk to sibs due to HLA = 1.49; observed value = 2.38). For hemochromatosis and Hodgkin's disease, there is little evidence for HLA-unlinked familial determinants. With this formula, it is also possible to examine the hypothesis of pleiotropy versus linkage dis-equilibrium by comparing lambda AS with the increased risk to sibs due to the associated allele(s).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbosa J., Bach F. H., Rich S. S. Genetic heterogeneity of diabetes and HLA. Clin Genet. 1982 Jan;21(1):25–32. doi: 10.1111/j.1399-0004.1982.tb02075.x. [DOI] [PubMed] [Google Scholar]
- Barbosa J., Chern M. M., Anderson V. E., Noreen H., Johnson S., Reinsmoen N., McCarty R., King R., Greenberg L. Linkage analysis between the major histocompatibility system and insulin-dependent diabetes in families with patients in two consecutive generations. J Clin Invest. 1980 Mar;65(3):592–601. doi: 10.1172/JCI109704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Horita S., Karam J. H. A polymorphic locus near the human insulin gene is associated with insulin-dependent diabetes mellitus. Diabetes. 1984 Feb;33(2):176–183. doi: 10.2337/diab.33.2.176. [DOI] [PubMed] [Google Scholar]
- Clerget-Darpoux F., Bonaiti-Pellie C., Hors J., Deschamps I., Feingold N. Application of the lod score method to detection of linkage between HLA and juvenile insulin-dependent diabetes. Clin Genet. 1980 Jul;18(1):51–57. doi: 10.1111/j.1399-0004.1980.tb01365.x. [DOI] [PubMed] [Google Scholar]
- Greenberg D. A., Hodge S. E., Rotter J. I. Evidence for recessive and against dominant inheritance at the HLA-"linked" locus in coeliac disease. Am J Hum Genet. 1982 Mar;34(2):263–277. [PMC free article] [PubMed] [Google Scholar]
- Haile R. W., Iselius L., Fine P. E., Morton N. E. Segregation and linkage analyses of 72 leprosy pedigrees. Hum Hered. 1985;35(1):43–52. doi: 10.1159/000153514. [DOI] [PubMed] [Google Scholar]
- Hattori M., Buse J. B., Jackson R. A., Glimcher L., Dorf M. E., Minami M., Makino S., Moriwaki K., Kuzuya H., Imura H. The NOD mouse: recessive diabetogenic gene in the major histocompatibility complex. Science. 1986 Feb 14;231(4739):733–735. doi: 10.1126/science.3003909. [DOI] [PubMed] [Google Scholar]
- Hitman G. A., Tarn A. C., Winter R. M., Drummond V., Williams L. G., Jowett N. I., Bottazzo G. F., Galton D. J. Type 1 (insulin-dependent) diabetes and a highly variable locus close to the insulin gene on chromosome 11. Diabetologia. 1985 Apr;28(4):218–222. doi: 10.1007/BF00282236. [DOI] [PubMed] [Google Scholar]
- Ho H. Z., Tiwari J. L., Haile R. W., Terasaki P. I., Morton N. E. HLA-linked and unlinked determinants of multiple sclerosis. Immunogenetics. 1982;15(5):509–517. doi: 10.1007/BF00345910. [DOI] [PubMed] [Google Scholar]
- Hodge S. E., Rotter J. I., Lange K. L. A three-allele model for heterogeneity of juvenile onset insulin-dependent diabetes. Ann Hum Genet. 1980 May;43(4):399–409. doi: 10.1111/j.1469-1809.1980.tb01573.x. [DOI] [PubMed] [Google Scholar]
- Hodge S. E. Some epistatic two-locus models of disease. I. Relative risks and identity-by-descent distributions in affected sib pairs. Am J Hum Genet. 1981 May;33(3):381–395. [PMC free article] [PubMed] [Google Scholar]
- Hors J., Dausset J. HLA and susceptibility to Hodgkin's disease. Immunol Rev. 1983;70:167–192. doi: 10.1111/j.1600-065x.1983.tb00714.x. [DOI] [PubMed] [Google Scholar]
- James J. W. Frequency in relatives for an all-or-none trait. Ann Hum Genet. 1971 Jul;35(1):47–49. doi: 10.1111/j.1469-1809.1956.tb01377.x. [DOI] [PubMed] [Google Scholar]
- Kravitz K., Skolnick M., Cannings C., Carmelli D., Baty B., Amos B., Johnson A., Mendell N., Edwards C., Cartwright G. Genetic linkage between hereditary hemochromatosis and HLA. Am J Hum Genet. 1979 Sep;31(5):601–619. [PMC free article] [PubMed] [Google Scholar]
- Kuwert E. K. Genetical aspects of multiple sclerosis with special regard to histocompatibility determinants. Acta Neurol Scand Suppl. 1977;63:23–42. [PubMed] [Google Scholar]
- Lalouel J. M., Le Mignon L., Simon M., Fauchet R., Bourel M., Rao D. C., Morton N. E. Genetic analysis of idiopathic hemochromatosis using both qualitative (disease status) and quantitative (serum iron) information. Am J Hum Genet. 1985 Jul;37(4):700–718. [PMC free article] [PubMed] [Google Scholar]
- MacLean C. J., Morton N. E., Yee S. Combined analysis of genetic segregation and linkage under an oligogenic model. Comput Biomed Res. 1984 Oct;17(5):471–480. doi: 10.1016/0010-4809(84)90013-2. [DOI] [PubMed] [Google Scholar]
- Payami H., Thomson G., Motro U., Louis E. J., Hudes E. The affected sib method. IV. Sib trios. Ann Hum Genet. 1985 Oct;49(Pt 4):303–314. doi: 10.1111/j.1469-1809.1985.tb01706.x. [DOI] [PubMed] [Google Scholar]
- Risch N. The effects of reduced fertility, method of ascertainment, and a second unlinked locus on affected sib-pair marker allele sharing. Am J Med Genet. 1983 Oct;16(2):243–259. doi: 10.1002/ajmg.1320160214. [DOI] [PubMed] [Google Scholar]
- Soudek D. Gregor Mendel and the people around him (commemorative of the centennial of Mendel's death). Am J Hum Genet. 1984 May;36(3):495–498. [PMC free article] [PubMed] [Google Scholar]
- Spielman R. S., Baker L., Zmijewski C. M. Gene dosage and suceptibility to insulin-dependent diabetes. Ann Hum Genet. 1980 Oct;44(Pt 2):135–150. doi: 10.1111/j.1469-1809.1980.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Spielman R. S., Nathanson N. The genetics of susceptibility to multiple sclerosis. Epidemiol Rev. 1982;4:45–65. doi: 10.1093/oxfordjournals.epirev.a036251. [DOI] [PubMed] [Google Scholar]
- Stewart G. J., McLeod J. G., Basten A., Bashir H. V. HLA family studies and multiple sclerosis: A common gene, dominantly expressed. Hum Immunol. 1981 Aug;3(1):13–29. doi: 10.1016/0198-8859(81)90040-9. [DOI] [PubMed] [Google Scholar]
- Suarez B., O'Rourke D., Van Eerdewegh P. Power of the affected-sib-pair method to defect disease susceptibility loci of small effect: an application to multiple sclerosis. Am J Med Genet. 1982 Jul;12(3):309–326. doi: 10.1002/ajmg.1320120309. [DOI] [PubMed] [Google Scholar]
- Svejgaard A., Ryder L. P. HLA genotype distribution and genetic models of insulin-dependent diabetes mellitus. Ann Hum Genet. 1981 Jul;45(Pt 3):293–298. doi: 10.1111/j.1469-1809.1981.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Thomson G. A two locus model for juvenile diabetes. Ann Hum Genet. 1980 May;43(4):383–398. doi: 10.1111/j.1469-1809.1980.tb01572.x. [DOI] [PubMed] [Google Scholar]
- Tiwari J. L., Betuel H., Gebuhrer L., Morton N. E. Genetic epidemiology of coeliac disease. Genet Epidemiol. 1984;1(1):37–42. doi: 10.1002/gepi.1370010106. [DOI] [PubMed] [Google Scholar]
- Wagener D. K., Sacks J. M., LaPorte R. E., Macgregor J. M. The Pittsburgh study of insulin-dependent diabetes mellitus. Risk for diabetes among relatives of IDDM. Diabetes. 1982 Feb;31(2):136–144. doi: 10.2337/diab.31.2.136. [DOI] [PubMed] [Google Scholar]


