Abstract
Ataxia telangiectasia is a genetically determined disease with multi-system abnormalities and a high incidence of neoplasia. In order to define the nature of the association between ataxia telangiectasia and malignancy, we investigated a patient with the disease and heterozygote for the Mediterranean variant of the X-linked marker glucose 6-phosphate dehydrogenase. Enzymatic mosaicism in hemopoietic and nonhemopoietic cells was evaluated with the 2-deoxy glucose 6-phosphate technique. While erythrocytes, platelets, and lymphocytes expressed the same double-enzyme phenotype as tissues of nonhemopoietic origin, granulocytes and monocytes expressed almost exclusively the Mediterranean-type enzyme. We suggest that, as the result of genetic instability at the hemopoietic stem-cell level, the granulocytic/monocytic progeny enjoyed a proliferative advantage and became the predominant clone.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fairbanks V. F., Lampe L. T. A tetrazolium-linked cytochemical method for estimation of glucose-6-phosphate dehydrogenase activity in individual erythrocytes: applications in the study of heterozygotes for glucose-6-phosphate dehydrogenase deficiency. Blood. 1968 May;31(5):589–603. [PubMed] [Google Scholar]
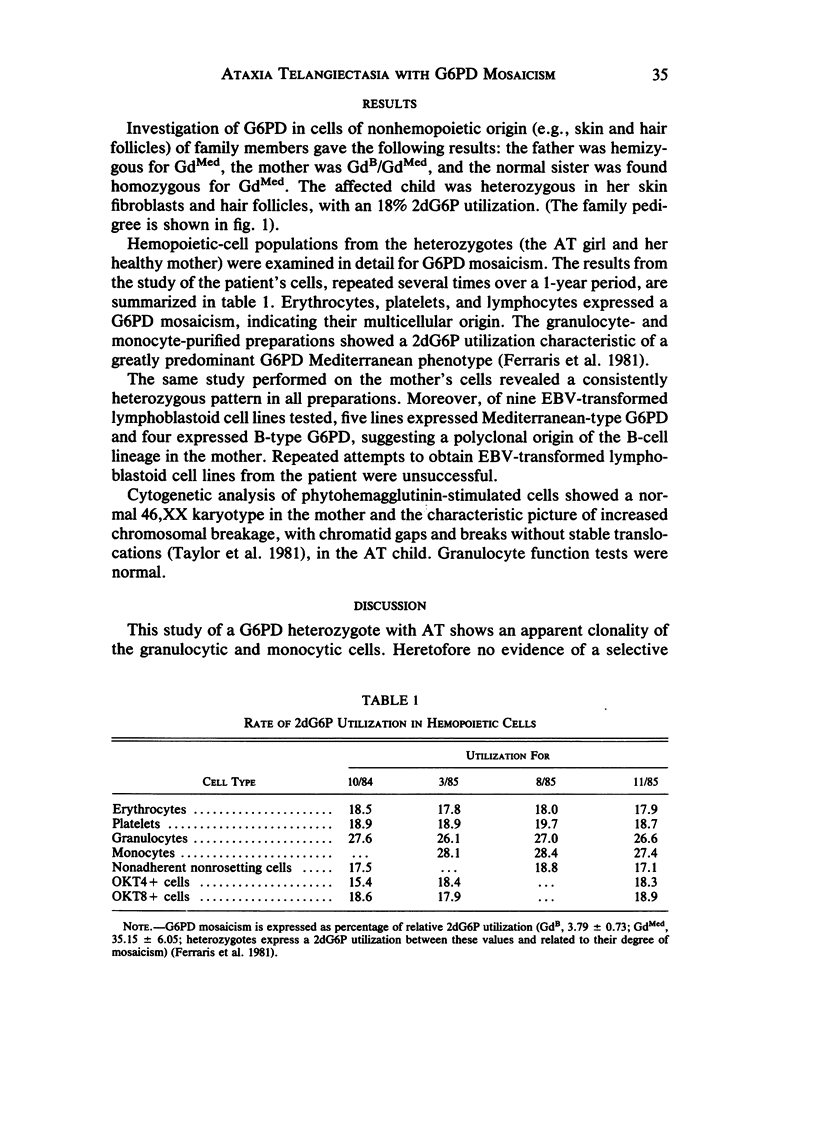
- Ferraris A. M., Broccia G., Meloni T., Canepa L., Sessarego M., Gaetani G. F. Clonal origin of cells restricted to monocytic differentiation in acute nonlymphocytic leukemia. Blood. 1984 Oct;64(4):817–820. [PubMed] [Google Scholar]
- Ferraris A. M., Canepa L., Mareni C., Baule G., Meloni T., Salvidio E., Forteleoni G., Gaetani G. F. Reexpression of normal stem cells in erythroleukemia during remission. Blood. 1983 Jul;62(1):177–179. [PubMed] [Google Scholar]
- Ferraris A. M., Canepa L., Massimo L., Dini G., Broccia G., Meloni T., Forteleoni G., Melani C., Gaetani G. F. Clonal development from a progenitor with restricted differentiative expression in acute lymphoblastic leukemia. Am J Hematol. 1985 Sep;20(1):81–83. doi: 10.1002/ajh.2830200111. [DOI] [PubMed] [Google Scholar]
- Ferraris A. M., Giuntini P., Galiano S., Gaetani G. F. 2-deoxy-glucose-6-phosphate utilization in the study of glucose-6-phosphate dehydrogenase mosaicism. Am J Hum Genet. 1981 Mar;33(2):307–313. [PMC free article] [PubMed] [Google Scholar]
- Ferraris A. M., Raskind W. H., Bjornson B. H., Jacobson R. J., Singer J. W., Fialkow P. J. Heterogeneity of B cell involvement in acute nonlymphocytic leukemia. Blood. 1985 Aug;66(2):342–344. [PubMed] [Google Scholar]
- Fialkow P. J. Clonal origin of human tumors. Biochim Biophys Acta. 1976 Oct 12;458(3):283–321. doi: 10.1016/0304-419x(76)90003-2. [DOI] [PubMed] [Google Scholar]
- Gaetani G. F., Ferraris A. M., Galiano S., Giuntini P., Canepa L., d'Urso M. Primary thrombocythemia: clonal origin of platelets, erythrocytes, and granulocytes in a GdB/GdMediterranean subject. Blood. 1982 Jan;59(1):76–79. [PubMed] [Google Scholar]
- Gealy W. J., Dwyer J. M., Harley J. B. Allelic exclusion of glucose-6-phosphate dehydrogenase in platelets and T lymphocytes from a Wiskott-Aldrich syndrome carrier. Lancet. 1980 Jan 12;1(8159):63–65. doi: 10.1016/s0140-6736(80)90492-4. [DOI] [PubMed] [Google Scholar]
- McKeran R. O., Howell A., Andrews T. M., Watts R. W., Arlett C. F. Observations on the growth in vitro of myeloid progenitor cells and fibroblasts from hemizygotes and heterozygotes for "complete" and "partial" hypoxanthine-guanine phosphoribosyltransferase (HGPRT) deficiency, and their relevance to the pathogenesis of brain damage in the Lesch-Nyhan syndrome. J Neurol Sci. 1974 Jun;22(2):183–195. doi: 10.1016/0022-510x(74)90245-7. [DOI] [PubMed] [Google Scholar]
- Packard B. S., Tavassoli M., Dale G. L., Beutler E. A method for the establishment and long-term maintenance of in vitro monocytic cultures with functional and morphological homogeneity. Blood. 1982 Sep;60(3):623–626. [PubMed] [Google Scholar]
- Peterson R. D., Cooper M. D., Good R. A. Lymphoid tissue abnormalities associated with ataxia-telangiectasia. Am J Med. 1966 Sep;41(3):342–359. doi: 10.1016/0002-9343(66)90080-5. [DOI] [PubMed] [Google Scholar]
- Raskind W. H., Tirumali N., Jacobson R., Singer J., Fialkow P. J. Evidence for a multistep pathogenesis of a myelodysplastic syndrome. Blood. 1984 Jun;63(6):1318–1323. [PubMed] [Google Scholar]
- Swift M. Genetics and epidemiology of ataxia-telangiectasia. Kroc Found Ser. 1985;19:133–146. [PubMed] [Google Scholar]
- Swift M., Sholman L., Perry M., Chase C. Malignant neoplasms in the families of patients with ataxia-telangiectasia. Cancer Res. 1976 Jan;36(1):209–215. [PubMed] [Google Scholar]
- Taylor A. M., Metcalfe J. A., Oxford J. M., Harnden D. G. Is chromatid-type damage in ataxia telangiectasia after irradiation at G0 a consequence of defective repair? Nature. 1976 Apr 1;260(5550):441–443. doi: 10.1038/260441a0. [DOI] [PubMed] [Google Scholar]
- Taylor A. M., Oxford J. M., Metcalfe J. A. Spontaneous cytogenetic abnormalities in lymphocytes from thirteen patients with ataxia telangiectasia. Int J Cancer. 1981 Mar 15;27(3):311–319. doi: 10.1002/ijc.2910270309. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Feinberg A. P. Use of restriction fragment length polymorphisms to determine the clonal origin of human tumors. Science. 1985 Feb 8;227(4687):642–645. doi: 10.1126/science.2982210. [DOI] [PubMed] [Google Scholar]
- Zarling J. M., Clouse K. A., Biddison W. E., Kung P. C. Phenotypes of human natural killer cell populations detected with monoclonal antibodies. J Immunol. 1981 Dec;127(6):2575–2580. [PubMed] [Google Scholar]


