Abstract
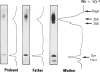
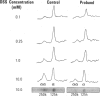
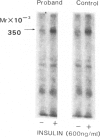
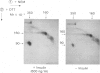
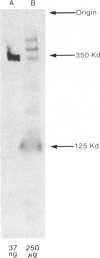
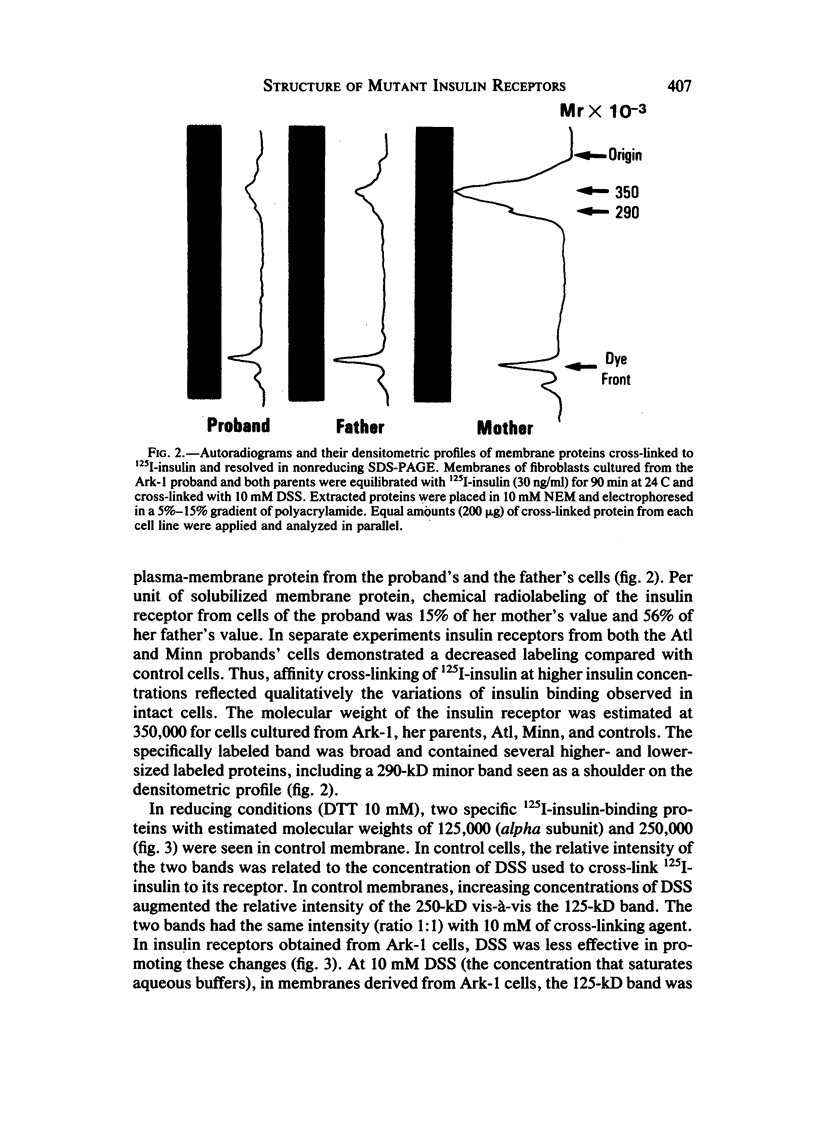
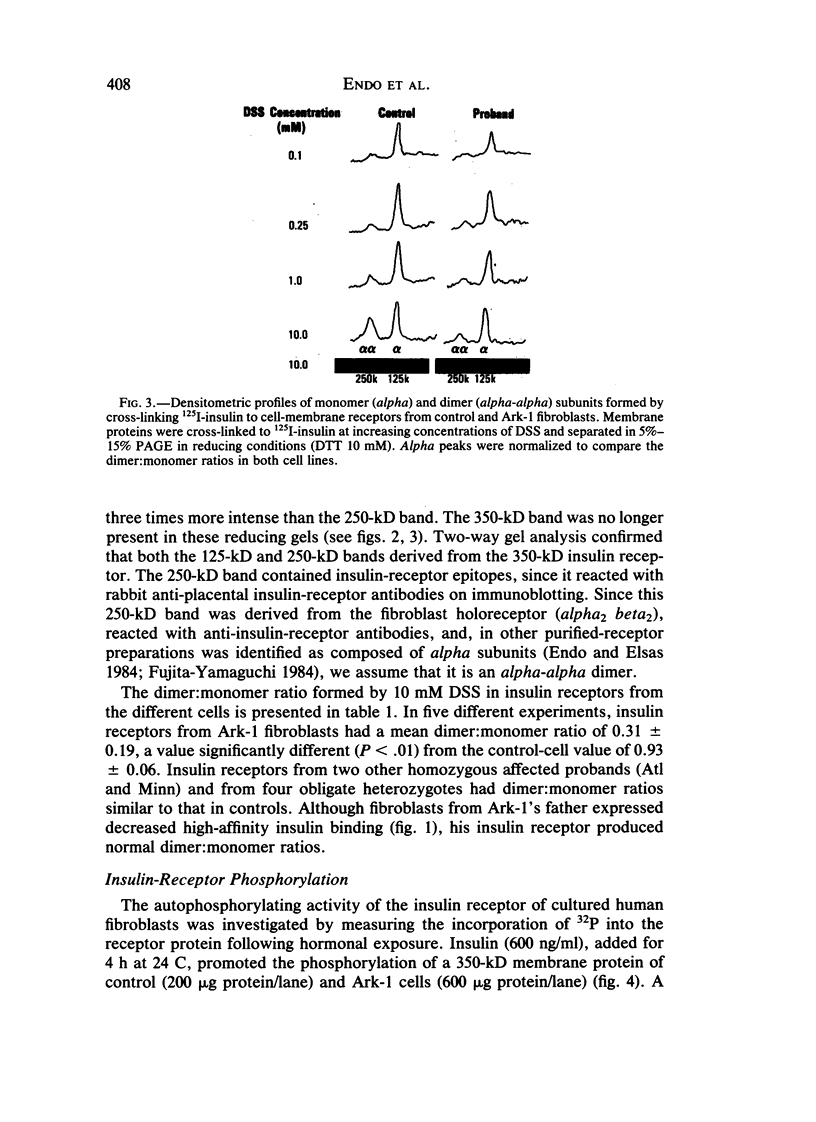
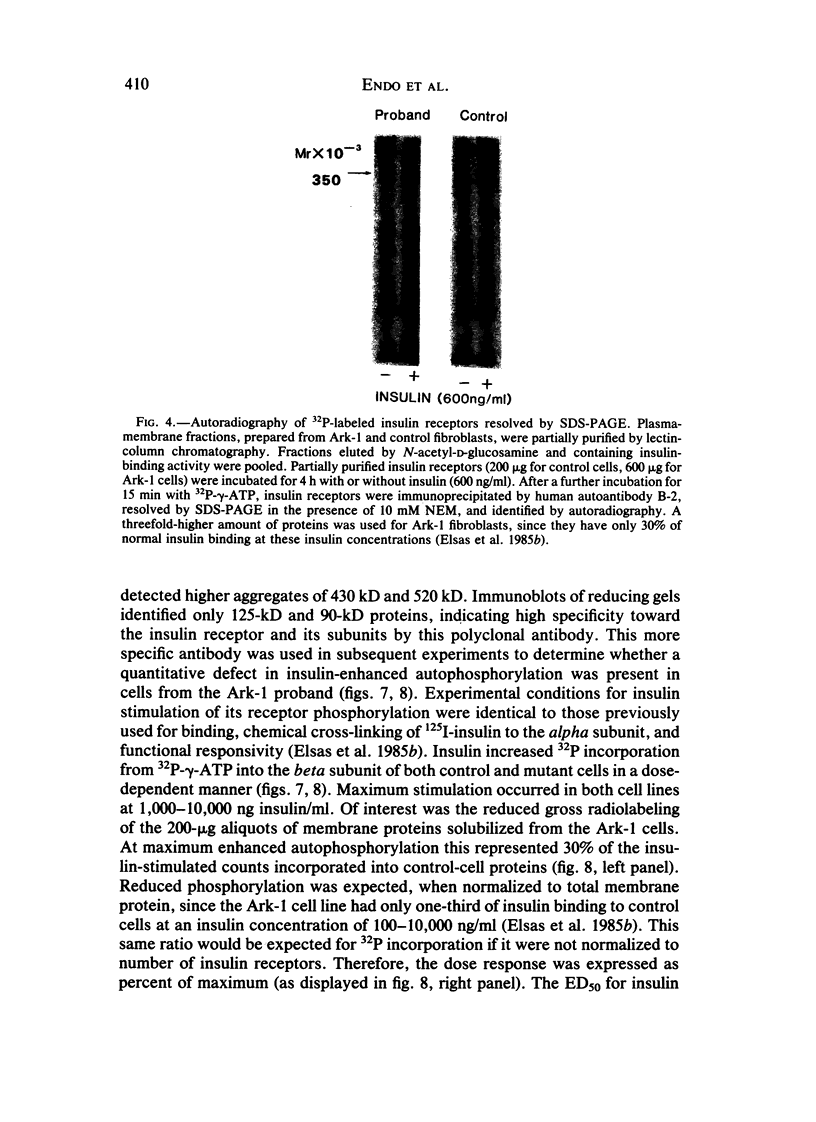
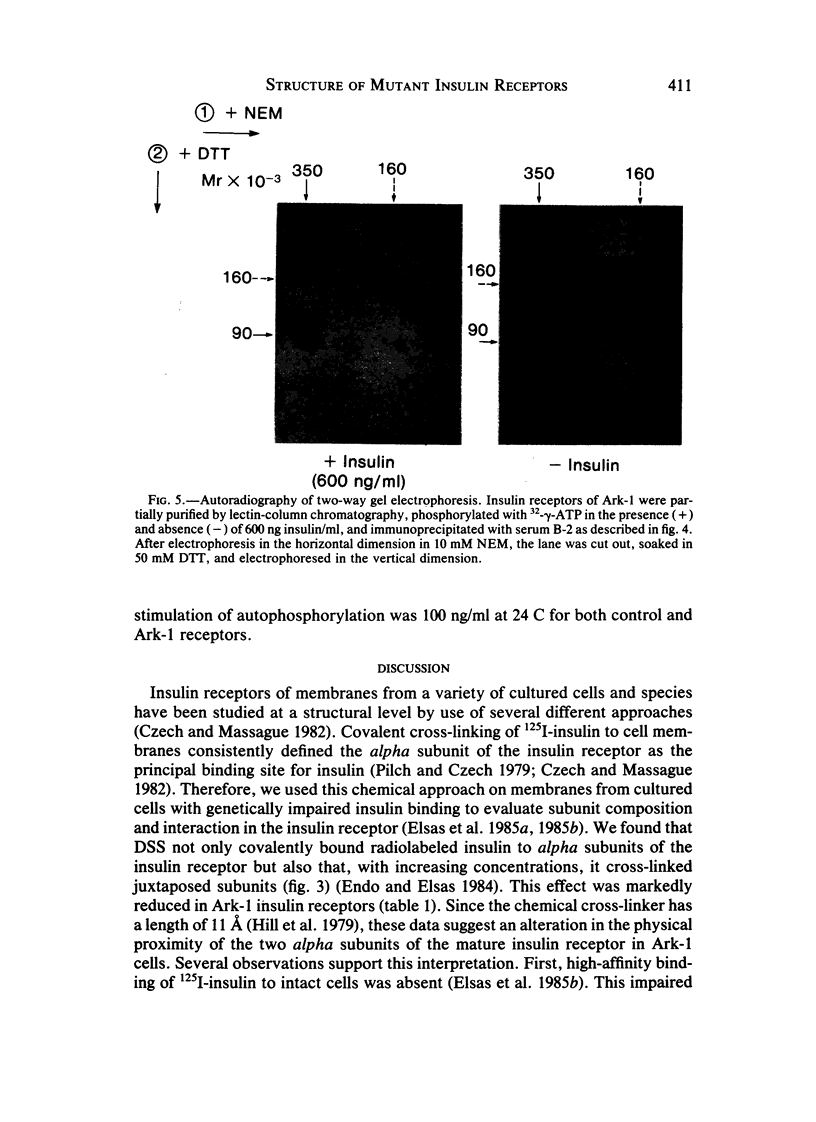
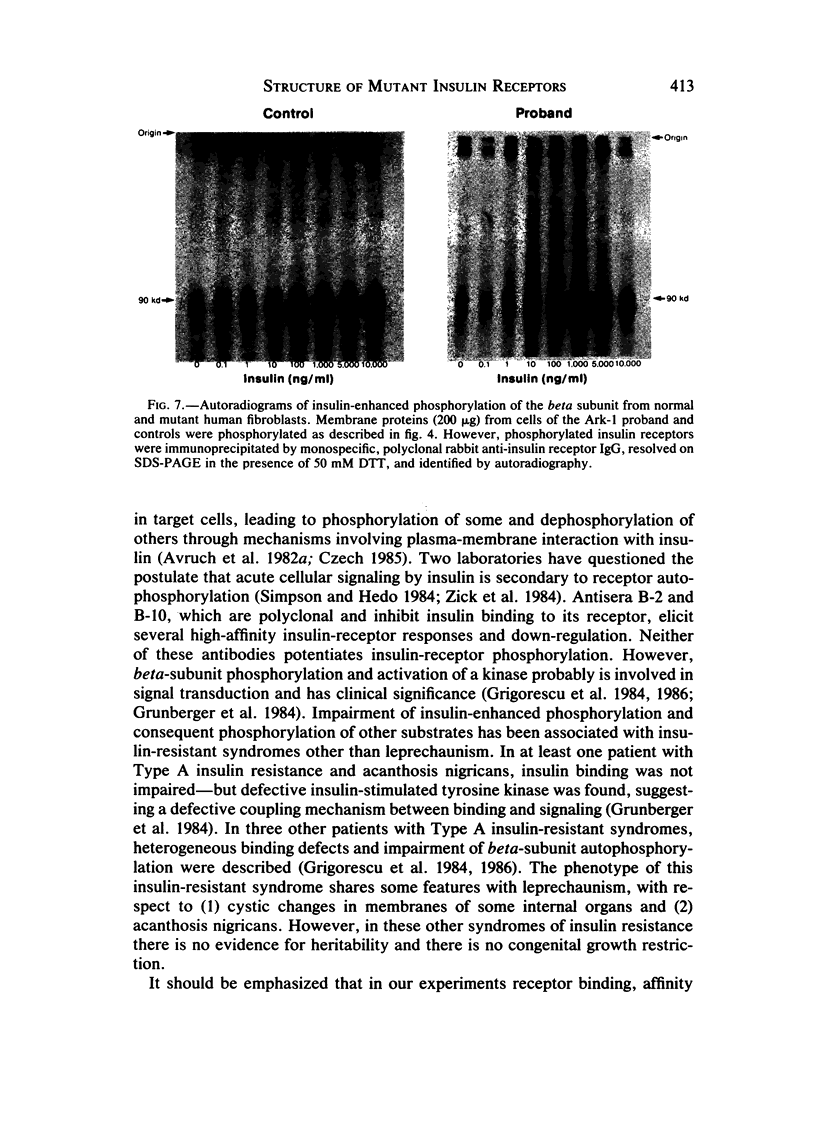
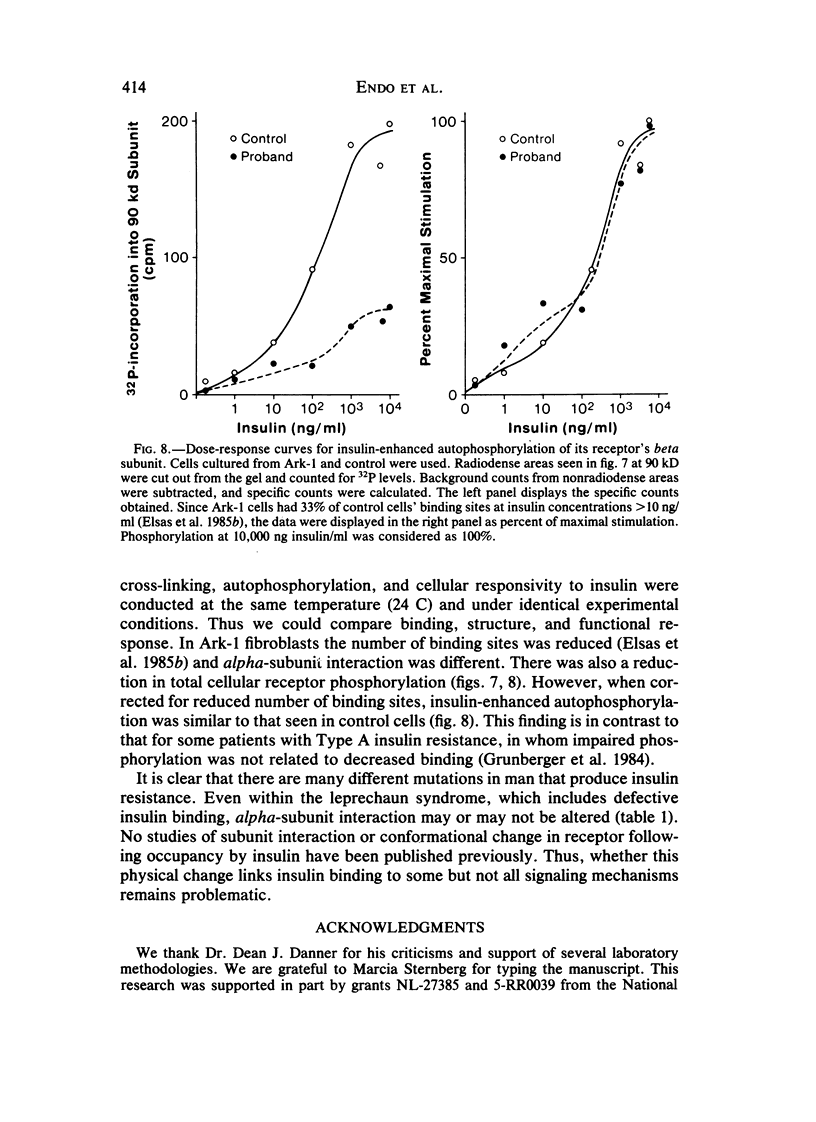
Leprechaunism is an inherited disorder characterized by insulin resistance and intrauterine growth restriction. In this study we analyze insulin binding and subunit structure of the insulin receptor in dermal fibroblasts cultured from three unrelated families whose probands (Ark-1, Atl, and Minn) were affected by leprechaunism. Cells cultured from all three probands had markedly reduced insulin binding at equilibrium. Fibroblasts cultured from the parents of Ark-1 and Atl had partial and differing degrees of impairment in insulin binding. The structure of the alpha subunit of insulin receptors was analyzed by cross-linking 125I-insulin to plasma membranes. A major band of 350 kilodaltons (kD) (corresponding to the heterotetrameric insulin receptor alpha 2 beta 2) was observed in control and leprechaun fibroblasts. The relative amount of radioactivity cross-linked to plasma membranes reflected the genetic variations seen in insulin binding to intact cells. In reducing gels, 125I-insulin was cross-linked equally to a 250-kD (alpha-alpha dimer) and a 125-kD (alpha monomer) protein in cells from controls, the parents of Ark-1 and Atl, and probands Atl and Minn. By contrast, cells from the Ark-1 proband had diminished cross-linking of alpha-alpha dimers. The ratio of dimer to monomer in cells from controls was 0.93 +/- 0.06, and that in cells from Ark-1 was 0.31 +/- 0.19 (P less than .01). Beta-subunit structure and function was analyzed by studying insulin-enhanced autophosphorylation. Although maximal stimulation of beta-subunit phosphorylation was reduced to 30% in proband Ark-1 fibroblasts, this reduction was quantitatively related to reduced insulin binding. These results indicate that mutations causing severe insulin resistance and defective insulin binding are transmitted with autosomal recessive patterns of inheritance and that heterogeneity exists for these mutations. The mutation in pedigree Ark-1 most likely produces conformational changes in alpha-subunit interaction.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Alexander M. C., Palmer J. L., Pierce M. W., Nemenoff R. A., Blackshear P. J., Tipper J. P., Witters L. A. Role of insulin-stimulated protein phosphorylation in insulin action. Fed Proc. 1982 Aug;41(10):2629–2633. [PubMed] [Google Scholar]
- Avruch J., Nemenoff R. A., Blackshear P. J., Pierce M. W., Osathanondh R. Insulin-stimulated tyrosine phosphorylation of the insulin receptor in detergent extracts of human placental membranes. Comparison to epidermal growth factor-stimulated phosphorylation. J Biol Chem. 1982 Dec 25;257(24):15162–15166. [PubMed] [Google Scholar]
- Craig J. W., Larner J., Locker E. F., Widom B., Elders M. J. Mechanisms of insulin resistance in cultured fibroblasts from a patient with leprechaunism: impaired post-binding actions of insulin and multiplication-stimulating activity. Metabolism. 1984 Dec;33(12):1084–1096. doi: 10.1016/0026-0495(84)90092-1. [DOI] [PubMed] [Google Scholar]
- Czech M. P., Massague J. Subunit structure and dynamics of the insulin receptor. Fed Proc. 1982 Sep;41(11):2719–2723. [PubMed] [Google Scholar]
- D'Ercole A. J., Underwood L. E., Groelke J., Plet A. Leprechaunism: studies of the relationship among hyperinsulinism, insulin resistance, and growth retardation. J Clin Endocrinol Metab. 1979 Mar;48(3):495–502. doi: 10.1210/jcem-48-3-495. [DOI] [PubMed] [Google Scholar]
- DONOHUE W. L., UCHIDA I. Leprechaunism: a euphemism for a rare familial disorder. J Pediatr. 1954 Nov;45(5):505–519. doi: 10.1016/s0022-3476(54)80113-2. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Elsas L. J., Endo F., Strumlauf E., Elders J., Priest J. H. Leprechaunism: an inherited defect in a high-affinity insulin receptor. Am J Hum Genet. 1985 Jan;37(1):73–88. [PMC free article] [PubMed] [Google Scholar]
- Endo F., Elsas L. J., 2nd Structural analysis and subunit interaction of insulin receptor from membranes of cultured embryonic chick heart cells. Endocrinology. 1984 Nov;115(5):1828–1837. doi: 10.1210/endo-115-5-1828. [DOI] [PubMed] [Google Scholar]
- Fujita-Yamaguchi Y. Characterization of purified insulin receptor subunits. J Biol Chem. 1984 Jan 25;259(2):1206–1211. [PubMed] [Google Scholar]
- Grigorescu F., Flier J. S., Kahn C. R. Defect in insulin receptor phosphorylation in erythrocytes and fibroblasts associated with severe insulin resistance. J Biol Chem. 1984 Dec 25;259(24):15003–15006. [PubMed] [Google Scholar]
- Grunberger G., Zick Y., Gorden P. Defect in phosphorylation of insulin receptors in cells from an insulin-resistant patient with normal insulin binding. Science. 1984 Mar 2;223(4639):932–934. doi: 10.1126/science.6141638. [DOI] [PubMed] [Google Scholar]
- Hill M., Bechet J. J., d'Albis A. Disuccinimidyl esters as bifunctional crosslinking reagents for proteins: assays with myosin. FEBS Lett. 1979 Jun 15;102(2):282–286. doi: 10.1016/0014-5793(79)80019-8. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Baird K., Filier J. S., Jarrett D. B. Effects of autoantibodies to the insulin receptor on isolated adipocytes. Studies of insulin binding and insulin action. J Clin Invest. 1977 Nov;60(5):1094–1106. doi: 10.1172/JCI108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Fujita-Yamaguchi Y., Blithe D. L., Kahn C. R. Tyrosine-specific protein kinase activity is associated with the purified insulin receptor. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2137–2141. doi: 10.1073/pnas.80.8.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Olefsky J. M., Elders J., Mako M. E., Given B. D., Schedwie H. K., Fiser R. H., Hintz R. L., Horner J. A., Rubenstein A. H. Insulin resistance due to a defect distal to the insulin receptor: demonstration in a patient with leprechaunism. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3469–3473. doi: 10.1073/pnas.75.7.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nemenoff R. A., Kwok Y. C., Shulman G. I., Blackshear P. J., Osathanondh R., Avruch J. Insulin-stimulated tyrosine protein kinase. Characterization and relation to the insulin receptor. J Biol Chem. 1984 Apr 25;259(8):5058–5065. [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. Interaction of cross-linking agents with the insulin effector system of isolated fat cells. Covalent linkage of 125I-insulin to a plasma membrane receptor protein of 140,000 daltons. J Biol Chem. 1979 May 10;254(9):3375–3381. [PubMed] [Google Scholar]
- Podskalny J. M., Kahn C. R. Cell culture studies on patients with extreme insulin resistance. I. Receptor defects on cultured fibroblasts. J Clin Endocrinol Metab. 1982 Feb;54(2):261–268. doi: 10.1210/jcem-54-2-261. [DOI] [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Rechler M. M. Leprechaunism and related syndromes with primary insulin resistance: heterogeneity of molecular defects. Prog Clin Biol Res. 1982;97:245–281. [PubMed] [Google Scholar]
- Rosen O. M., Herrera R., Olowe Y., Petruzzelli L. M., Cobb M. H. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3237–3240. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. A., Cassell D. J. Insulin receptor: evidence that it is a protein kinase. Science. 1983 Jan 21;219(4582):299–301. doi: 10.1126/science.6849137. [DOI] [PubMed] [Google Scholar]
- Santora A. C., 2nd, Wheeler F. B., DeHaan R. L., Elsas L. J., 2nd Relationship of insulin binding to amino acid transport by cultured 14-day embryonic chick heart cells. Endocrinology. 1979 Apr;104(4):1059–1068. doi: 10.1210/endo-104-4-1059. [DOI] [PubMed] [Google Scholar]
- Schilling E. E., Rechler M. M., Grunfeld C., Rosenberg A. M. Primary defect of insulin receptors in skin fibroblasts cultured from an infant with leprechaunism and insulin resistance. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5877–5881. doi: 10.1073/pnas.76.11.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shia M. A., Rubin J. B., Pilch P. F. The insulin receptor protein kinase. Physicochemical requirements for activity. J Biol Chem. 1983 Dec 10;258(23):14450–14455. [PubMed] [Google Scholar]
- Simpson I. A., Hedo J. A. Insulin receptor phosphorylation may not be a prerequisite for acute insulin action. Science. 1984 Mar 23;223(4642):1301–1304. doi: 10.1126/science.6367041. [DOI] [PubMed] [Google Scholar]
- Taylor S. I., Marcus-Samuels B., Ryan-Young J., Leventhal S., Elders M. J. Genetics of the insulin receptor defect in a patient with extreme insulin resistance. J Clin Endocrinol Metab. 1986 Jun;62(6):1130–1135. doi: 10.1210/jcem-62-6-1130. [DOI] [PubMed] [Google Scholar]
- Taylor S. I. Receptor defects in patients with extreme insulin resistance. Diabetes Metab Rev. 1985;1(1-2):171–202. doi: 10.1002/dmr.5610010109. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Van Obberghen E., Rossi B., Kowalski A., Gazzano H., Ponzio G. Receptor-mediated phosphorylation of the hepatic insulin receptor: evidence that the Mr 95,000 receptor subunit is its own kinase. Proc Natl Acad Sci U S A. 1983 Feb;80(4):945–949. doi: 10.1073/pnas.80.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven G. F., Wilson J. D. The syndromes of primary hormone resistance. Metabolism. 1979 Mar;28(3):253–289. doi: 10.1016/0026-0495(79)90072-6. [DOI] [PubMed] [Google Scholar]
- Yang-Feng T. L., Francke U., Ullrich A. Gene for human insulin receptor: localization to site on chromosome 19 involved in pre-B-cell leukemia. Science. 1985 May 10;228(4700):728–731. doi: 10.1126/science.3873110. [DOI] [PubMed] [Google Scholar]
- Zick Y., Kasuga M., Kahn C. R., Roth J. Characterization of insulin-mediated phosphorylation of the insulin receptor in a cell-free system. J Biol Chem. 1983 Jan 10;258(1):75–80. [PubMed] [Google Scholar]
- Zick Y., Rees-Jones R. W., Taylor S. I., Gorden P., Roth J. The role of antireceptor antibodies in stimulating phosphorylation of the insulin receptor. J Biol Chem. 1984 Apr 10;259(7):4396–4400. [PubMed] [Google Scholar]