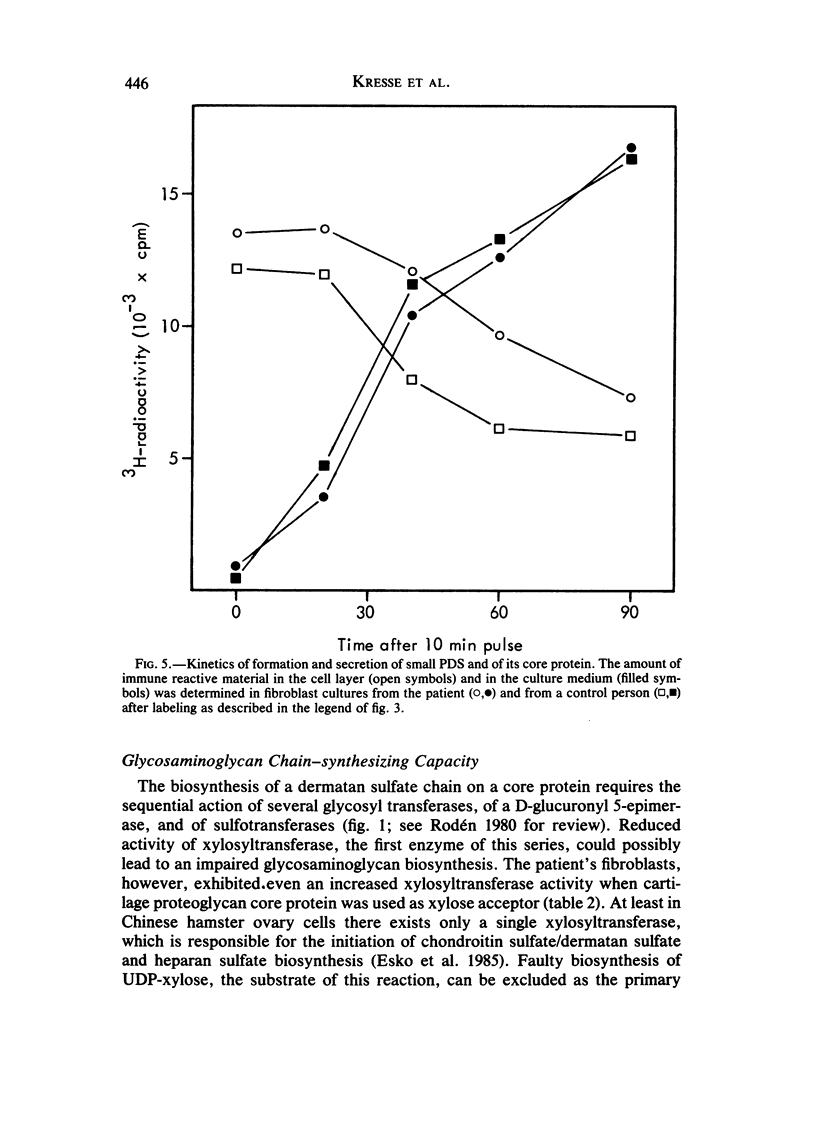
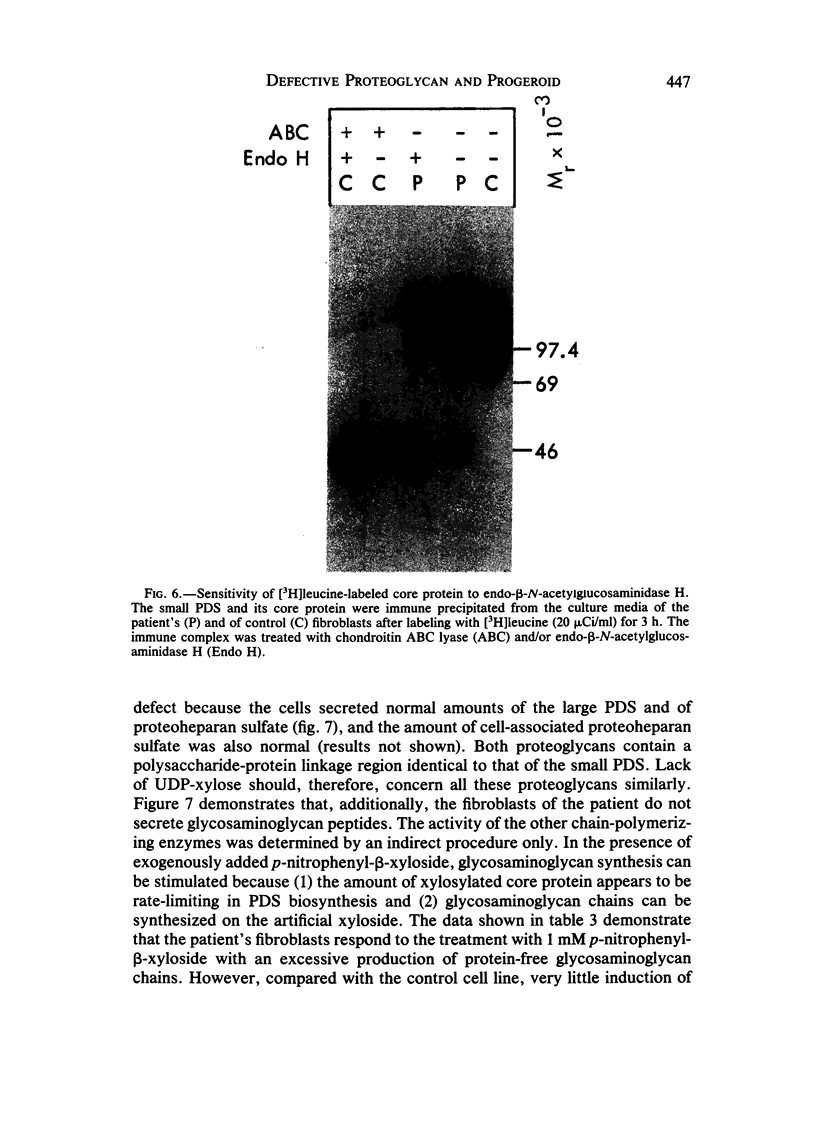
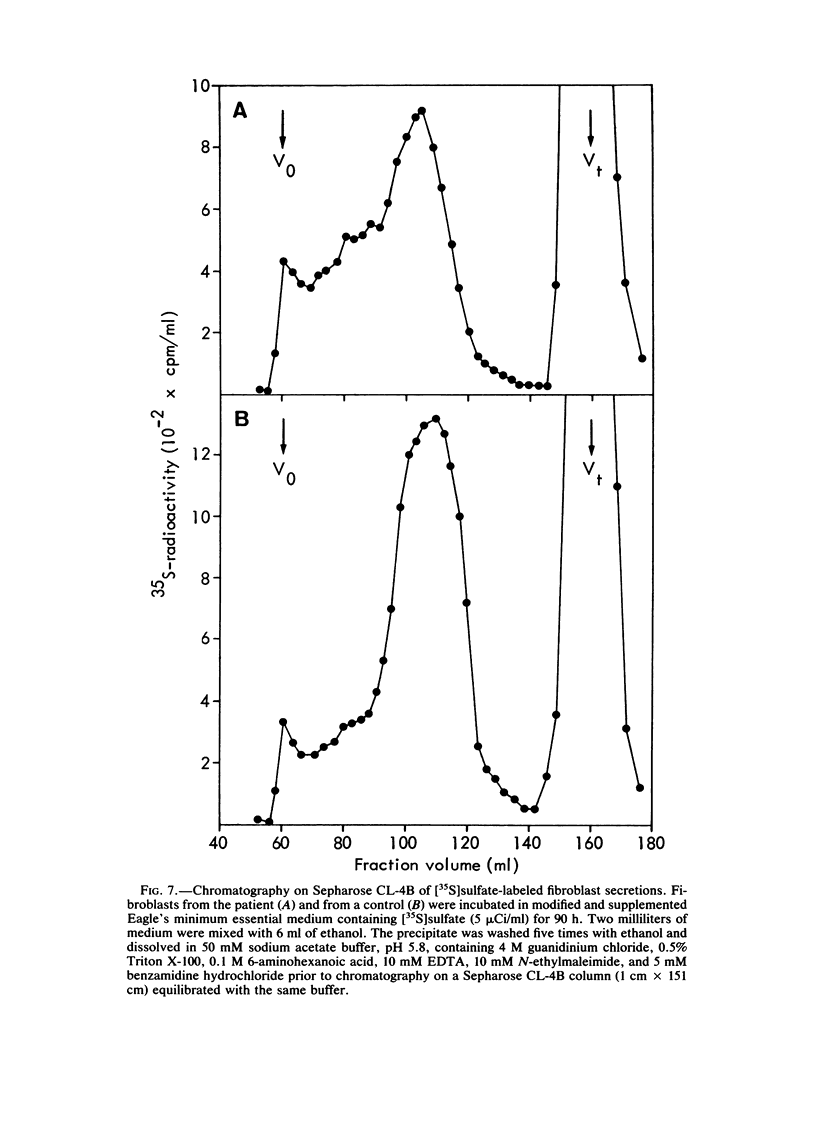
Abstract
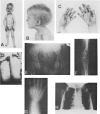
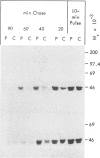
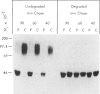
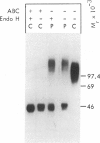
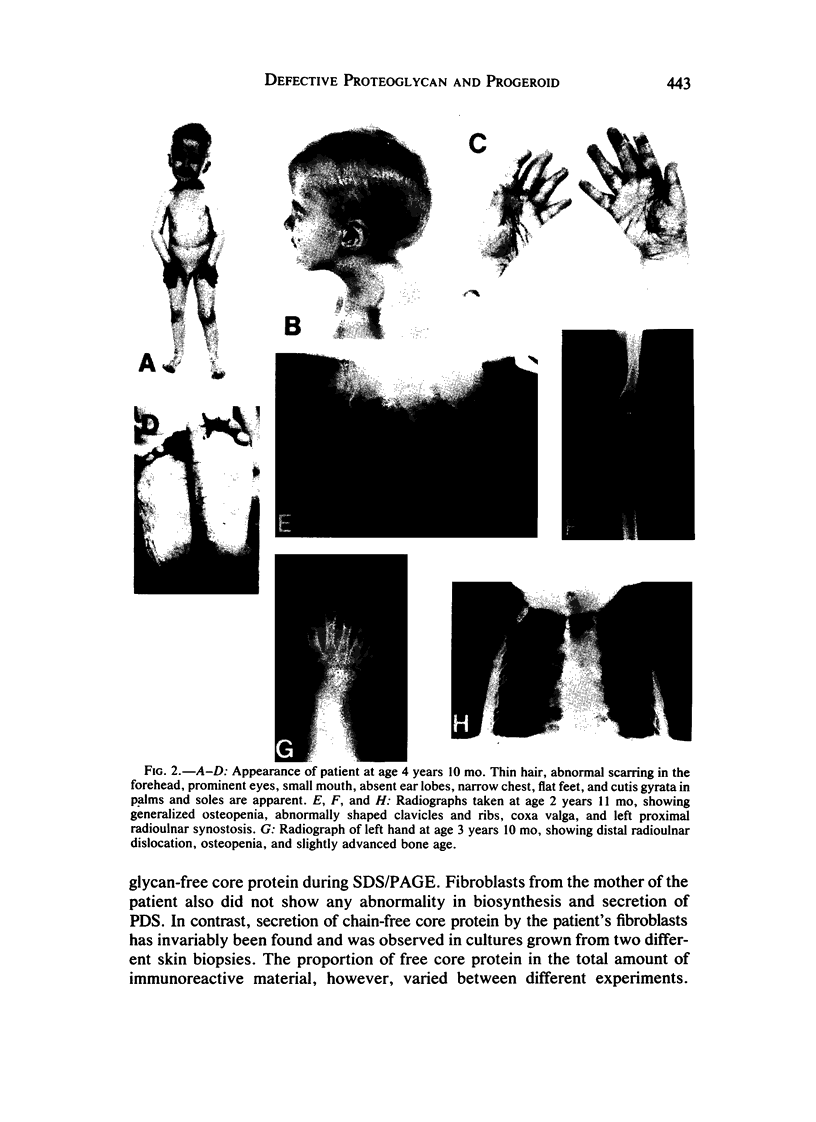
A male patient, 4 years 9 mo old and having progeroidal appearance, exhibited delayed mental development and multiple abnormalities of connective tissues including growth failure, osteopenia of all and dysplasia of some bones, defective deciduous teeth, loose but elastic skin, delayed wound healing with formation of thin atrophic scars, scanty scalp hair, hypotonic muscles, and hypermobile joints. Skin fibroblasts of the patient converted only about half of the core protein of the small proteodermatan sulfate to a mature glycosaminoglycan chain-bearing proteoglycan. The remaining core protein, which contained complex-type asparagine-bound oligosaccharides, was secreted with almost normal kinetics. Xylosyltransferase activity and the synthesis of other proteoglycan types were normal. Normal induction of glycosaminoglycan synthesis occurred in the presence of 1 mM, but there was very little induction in the presence of 0.01 mM p-nitrophenyl-beta-xyloside. An antibody against an N-terminal pentadecapeptide of the core protein recognized the glycosaminoglycan-free core protein from the patient less well than the chain-bearing protein treated with chondroitin ABC lyase. Though these results do not define the basic defect unambiguously, they provide the first report of a disorder being due to an abnormality in small proteoglycan biosynthesis.
Full text
PDF

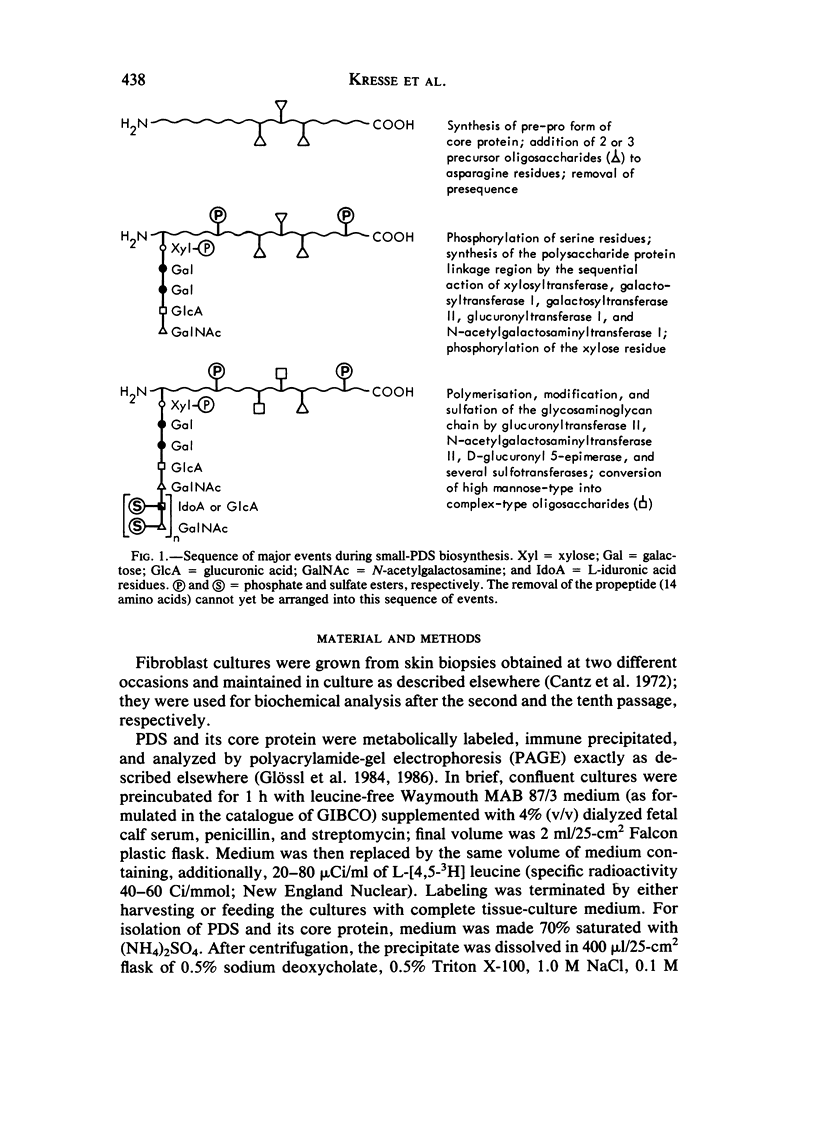




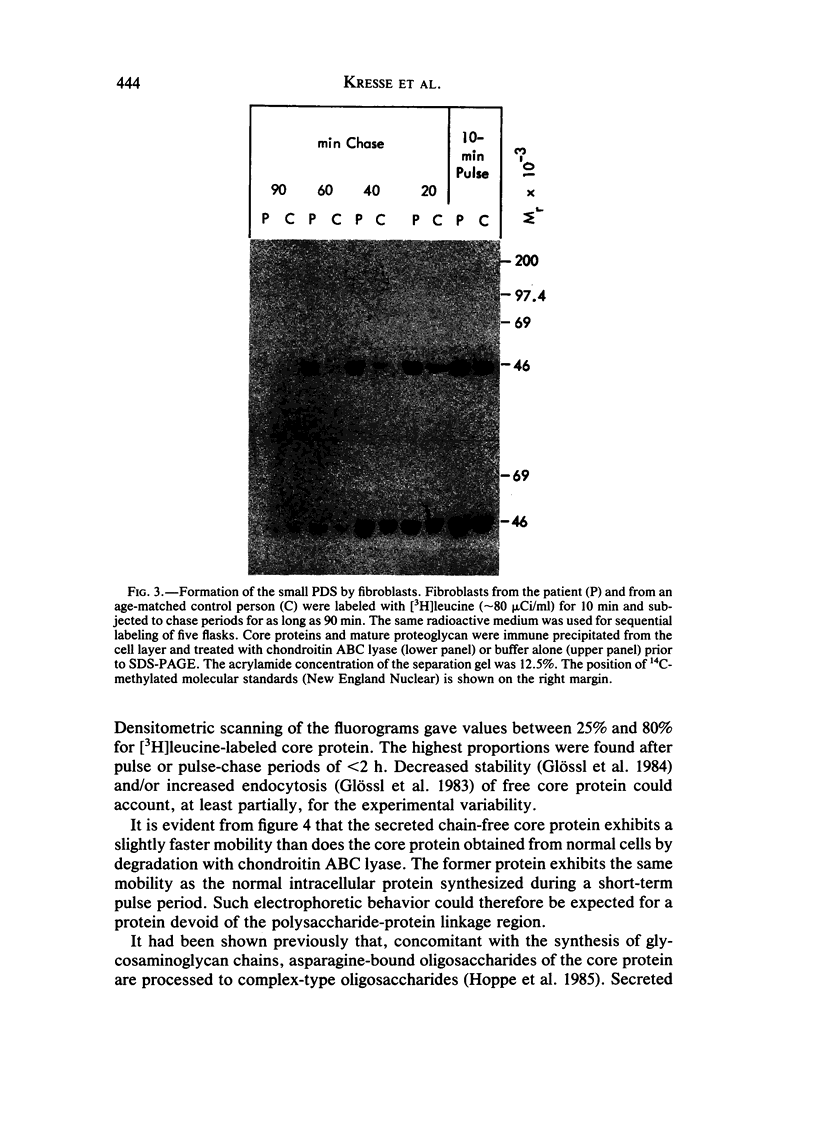
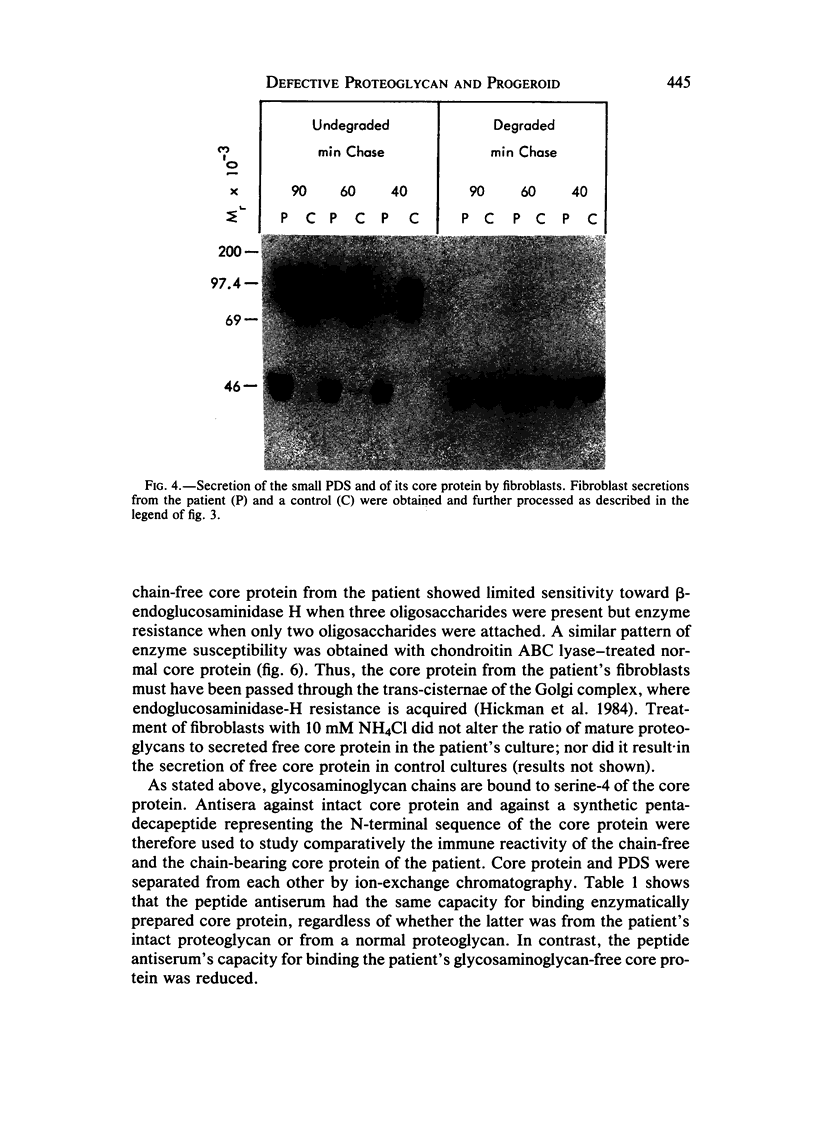





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOMMER W., KUENZER W., HAUSER W. [Disease picture with signs of progeria (Hutchinson-Gilford) and Ehlers-Danlos syndrome. (An unusual mensenchymal dysplasia with strong similarities to "Gottron's acrogeria")]. Arch Kinderheilkd. 1961 Dec;165:172–184. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cheah K. S. Collagen genes and inherited connective tissue disease. Biochem J. 1985 Jul 15;229(2):287–303. doi: 10.1042/bj2290287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra R. K., Pearson C. H., Pringle G. A., Fackre D. S., Scott P. G. Dermatan sulphate is located on serine-4 of bovine skin proteodermatan sulphate. Demonstration that most molecules possess only one glycosaminoglycan chain and comparison of amino acid sequences around glycosylation sites in different proteoglycans. Biochem J. 1985 Nov 15;232(1):277–279. doi: 10.1042/bj2320277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBusk F. L. The Hutchinson-Gilford progeria syndrome. Report of 4 cases and review of the literature. J Pediatr. 1972 Apr;80(4):697–724. doi: 10.1016/s0022-3476(72)80229-4. [DOI] [PubMed] [Google Scholar]
- Donnelly P. V., Reed P., DiFerrante N. Synthesis and sulfation of glycosaminoglycans in fibroblasts from a patient with Lowe's syndrome. Connect Tissue Res. 1984;13(1):89–98. doi: 10.3109/03008208409152146. [DOI] [PubMed] [Google Scholar]
- Esko J. D., Stewart T. E., Taylor W. H. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc Natl Acad Sci U S A. 1985 May;82(10):3197–3201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glössl J., Beck M., Kresse H. Biosynthesis of proteodermatan sulfate in cultured human fibroblasts. J Biol Chem. 1984 Nov 25;259(22):14144–14150. [PubMed] [Google Scholar]
- Glössl J., Hoppe W., Kresse H. Post-translational phosphorylation of proteodermatan sulfate. J Biol Chem. 1986 Feb 5;261(4):1920–1923. [PubMed] [Google Scholar]
- Glössl J., Schubert-Prinz R., Gregory J. D., Damle S. P., von Figura K., Kresse H. Receptor-mediated endocytosis of proteoglycans by human fibroblasts involves recognition of the protein core. Biochem J. 1983 Nov 1;215(2):295–301. doi: 10.1042/bj2150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Hassell J. R., Newsome D. A., Krachmer J. H., Rodrigues M. M. Macular corneal dystrophy: failure to synthesize a mature keratan sulfate proteoglycan. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3705–3709. doi: 10.1073/pnas.77.6.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman S., Theodorakis J. L., Greco J. M., Brown P. H. Processing of MOPC 315 immunoglobulin A oligosaccharides: evidence for endoplasmic reticulum and trans Golgi alpha 1,2-mannosidase activity. J Cell Biol. 1984 Feb;98(2):407–416. doi: 10.1083/jcb.98.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe W., Glössl J., Kresse H. Influence of monensin on biosynthesis, processing and secretion of proteodermatan sulfate by skin fibroblasts. Eur J Biochem. 1985 Oct 1;152(1):91–97. doi: 10.1111/j.1432-1033.1985.tb09167.x. [DOI] [PubMed] [Google Scholar]
- Krusius T., Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze J., Majewski F., Montgomery P., Hockey A., Karkut I., Riebel T. De Barsy syndrome--an autosomal recessive, progeroid syndrome. Eur J Pediatr. 1985 Nov;144(4):348–354. doi: 10.1007/BF00441776. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mourão P. A., Kato S., Donnelly P. V. Spondyloepiphyseal dysplasia, chondroitin sulfate type: a possible defect of PAPS--chondroitin sulfate sulfotransferase in humans. Biochem Biophys Res Commun. 1981 Jan 30;98(2):388–396. doi: 10.1016/0006-291x(81)90852-4. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Hassell J. R., Hascall V. C., Lohmander L. S., Newsome D. A., Krachmer J. Defective processing of keratan sulfate in macular corneal dystrophy. J Biol Chem. 1984 Nov 25;259(22):13751–13757. [PubMed] [Google Scholar]
- Olson C. A., Krueger R., Schwartz N. B. Deglycosylation of chondroitin sulfate proteoglycan by hydrogen fluoride in pyridine. Anal Biochem. 1985 Apr;146(1):232–237. doi: 10.1016/0003-2697(85)90420-8. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Heritable diseases of collagen. N Engl J Med. 1984 Aug 9;311(6):376–386. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Scott J. E., Haigh M. Proteoglycan-type I collagen fibril interactions in bone and non-calcifying connective tissues. Biosci Rep. 1985 Jan;5(1):71–81. doi: 10.1007/BF01117443. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Orford C. R. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem J. 1981 Jul 1;197(1):213–216. doi: 10.1042/bj1970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanescu V., Maroteaux P. Gel electrophoretic studies on proteoglycans and collagen of abnormal human growth cartilage: proteoglycan abnormalities in pseudoachondroplasia and in Kniest's disease. Pediatr Res. 1975 Oct;9(10):779–782. doi: 10.1203/00006450-197510000-00006. [DOI] [PubMed] [Google Scholar]
- Vogel K. G., Paulsson M., Heinegård D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984 Nov 1;223(3):587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss B., Glössl J., Cully Z., Kresse H. Immunocytochemical investigation on the distribution of small chondroitin sulfate-dermatan sulfate proteoglycan in the human. J Histochem Cytochem. 1986 Aug;34(8):1013–1019. doi: 10.1177/34.8.2426331. [DOI] [PubMed] [Google Scholar]
- Völker W., Schmidt A., Buddecke E., Themann H., Robenek H. Binding and degradation of proteoglycans by cultured arterial smooth muscle cells. II. Binding sites of proteoglycans on the cell surface. Eur J Cell Biol. 1985 Jan;36(1):58–65. [PubMed] [Google Scholar]
- Wiedemann H. R. Uber einige progeroid Krankheitsbilder und deren diagnostische Einordnung. Z Kinderheilkd. 1969;107(2):91–106. [PubMed] [Google Scholar]
- Yamashina I., Yoshida H., Fukui S., Funakoshi I. Biochemical studies on Lowe's syndrome. Mol Cell Biochem. 1983;52(2):107–124. doi: 10.1007/BF00224920. [DOI] [PubMed] [Google Scholar]