Abstract
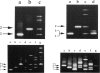
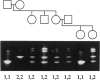
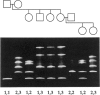
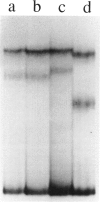
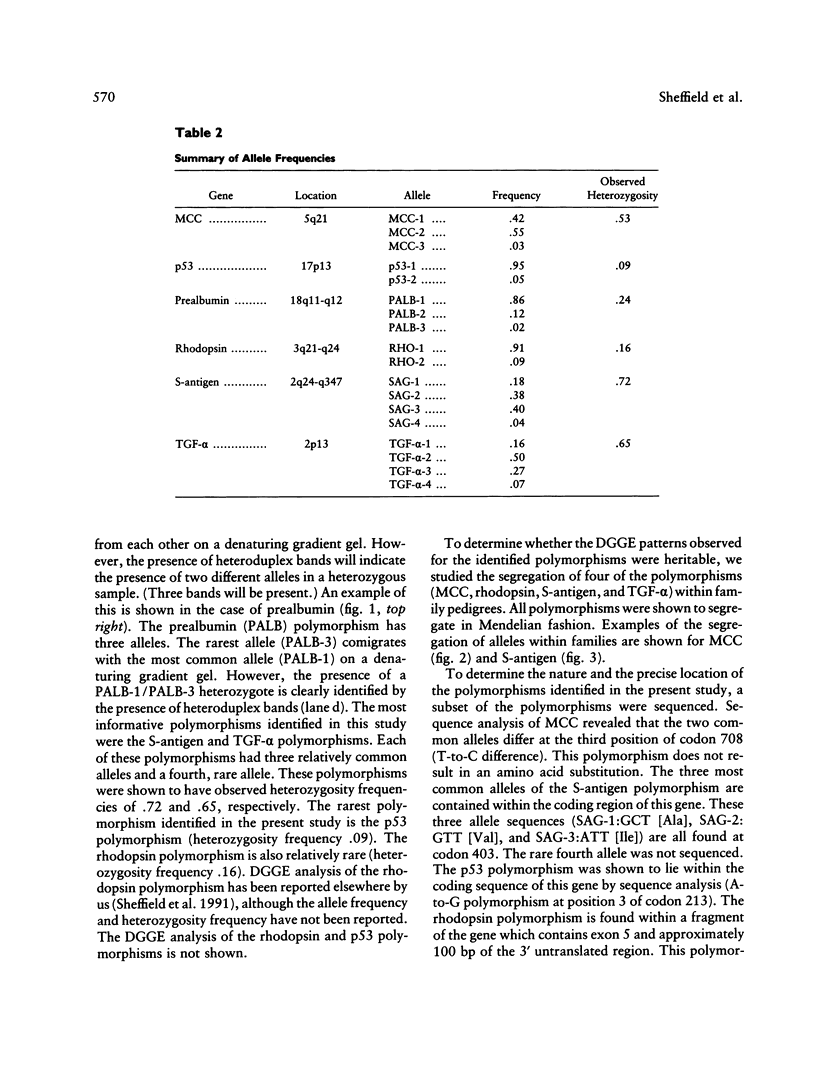
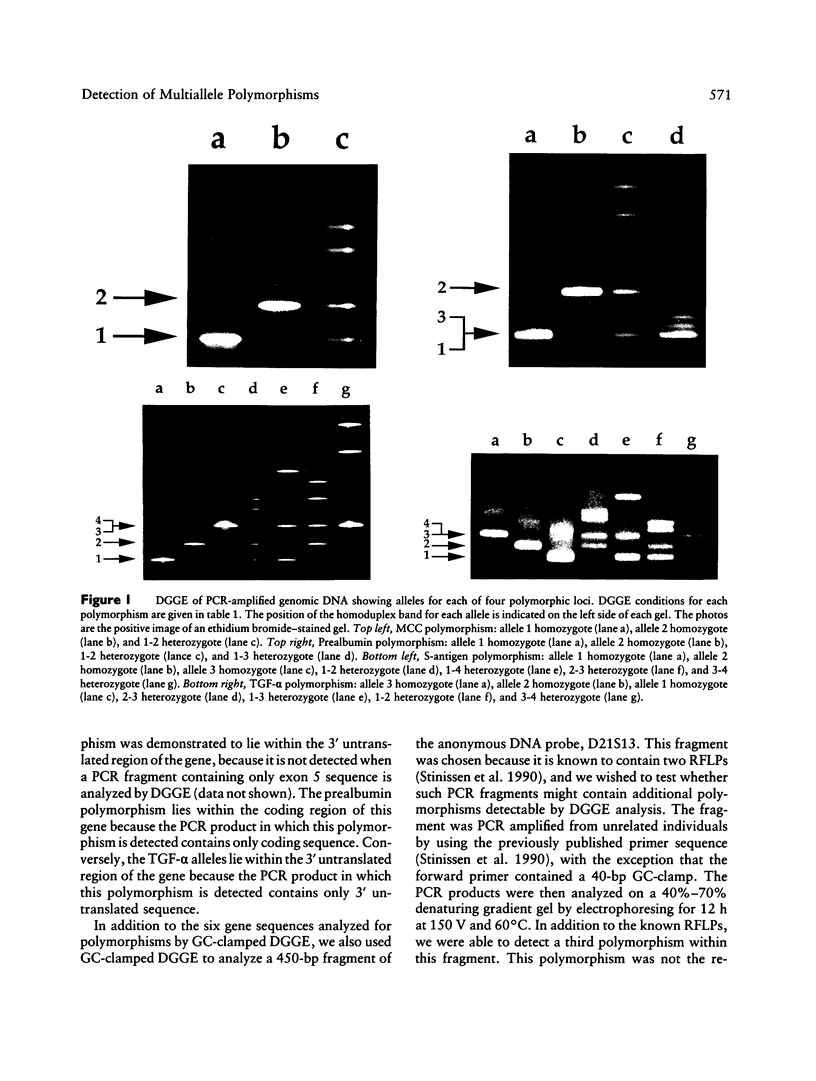
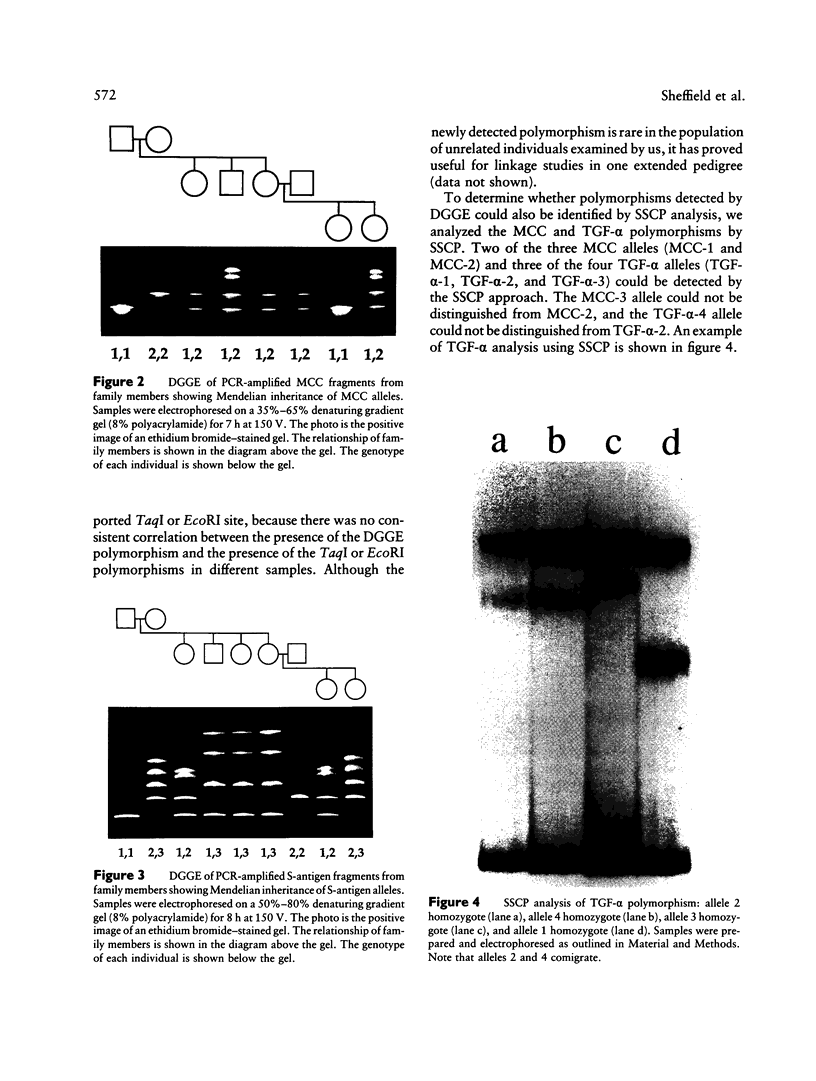
The ability to efficiently detect DNA polymorphisms is essential for the completion of a high-resolution polymorphic linkage map of the human genome. Currently the most informative polymorphisms are the multiallelic dinucleotide repeat polymorphisms. However, many gene sequences lack an associated dinucleotide repeat sequence. We used GC-clamped denaturing gradient gel electrophoresis to screen for DNA polymorphisms in the following six gene sequences: MCC, p53, prealbumin (transthyretin), rhodopsin, S-antigen, and TGF-alpha. A single-base sequence polymorphism was identified in each of these gene sequences. Some of these polymorphisms were multiallelic and highly informative. Our results demonstrate the value of denaturing gradient gel electrophoresis for both identifying and analyzing human DNA polymorphisms. The ability to detect highly informative polymorphisms within gene sequences will greatly contribute to a gene-based polymorphic linkage map.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Cooper D. N., Smith B. A., Cooke H. J., Niemann S., Schmidtke J. An estimate of unique DNA sequence heterozygosity in the human genome. Hum Genet. 1985;69(3):201–205. doi: 10.1007/BF00293024. [DOI] [PubMed] [Google Scholar]
- Cotton R. G. Detection of single base changes in nucleic acids. Biochem J. 1989 Oct 1;263(1):1–10. doi: 10.1042/bj2630001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBose R. F., Hartl D. L. Rapid purification of PCR products for DNA sequencing using Sepharose CL-6B spin columns. Biotechniques. 1990 Mar;8(3):271–274. [PubMed] [Google Scholar]
- Gray M., Charpentier A., Walsh K., Wu P., Bender W. Mapping point mutations in the Drosophila rosy locus using denaturing gradient gel blots. Genetics. 1991 Jan;127(1):139–149. doi: 10.1093/genetics/127.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofker M. H., Skraastad M. I., Bergen A. A., Wapenaar M. C., Bakker E., Millington-Ward A., van Ommen G. J., Pearson P. L. The X chromosome shows less genetic variation at restriction sites than the autosomes. Am J Hum Genet. 1986 Oct;39(4):438–451. [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., Tsui L. C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989 Sep 8;245(4922):1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Kinzler K. W., Nilbert M. C., Vogelstein B., Bryan T. M., Levy D. B., Smith K. J., Preisinger A. C., Hamilton S. R., Hedge P., Markham A. Identification of a gene located at chromosome 5q21 that is mutated in colorectal cancers. Science. 1991 Mar 15;251(4999):1366–1370. doi: 10.1126/science.1848370. [DOI] [PubMed] [Google Scholar]
- Lamb P., Crawford L. Characterization of the human p53 gene. Mol Cell Biol. 1986 May;6(5):1379–1385. doi: 10.1128/mcb.6.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Massagué J. Transforming growth factor-alpha. A model for membrane-anchored growth factors. J Biol Chem. 1990 Dec 15;265(35):21393–21396. [PubMed] [Google Scholar]
- Metzger A. K., Sheffield V. C., Duyk G., Daneshvar L., Edwards M. S., Cogen P. H. Identification of a germ-line mutation in the p53 gene in a patient with an intracranial ependymoma. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7825–7829. doi: 10.1073/pnas.88.17.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M. M., Koop B. F., Slightom J. L., Goodman M., Tennant M. R. Molecular systematics of higher primates: genealogical relations and classification. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7627–7631. doi: 10.1073/pnas.85.20.7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco A. P., Neve R. L., Colletti-Feener C., Bertelson C. J., Kurnit D. M., Kunkel L. M. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature. 1986 Oct 16;323(6089):646–650. doi: 10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- Murray J. C., Mills K. A., Demopulos C. M., Hornung S., Motulsky A. G. Linkage disequilibrium and evolutionary relationships of DNA variants (restriction enzyme fragment length polymorphisms) at the serum albumin locus. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3486–3490. doi: 10.1073/pnas.81.11.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Fischer S. G., Lerman L. S., Maniatis T. Nearly all single base substitutions in DNA fragments joined to a GC-clamp can be detected by denaturing gradient gel electrophoresis. Nucleic Acids Res. 1985 May 10;13(9):3131–3145. doi: 10.1093/nar/13.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Maniatis T., Lerman L. S. Detection and localization of single base changes by denaturing gradient gel electrophoresis. Methods Enzymol. 1987;155:501–527. doi: 10.1016/0076-6879(87)55033-9. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Maniatis T. Recent advances in the development of methods for detecting single-base substitutions associated with human genetic diseases. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):275–284. doi: 10.1101/sqb.1986.051.01.033. [DOI] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. Isolation and nucleotide sequence of the gene encoding human rhodopsin. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4851–4855. doi: 10.1073/pnas.81.15.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols W. C., Benson M. D. Hereditary amyloidosis: detection of variant prealbumin genes by restriction enzyme analysis of amplified genomic DNA sequences. Clin Genet. 1990 Jan;37(1):44–53. doi: 10.1111/j.1399-0004.1990.tb03389.x. [DOI] [PubMed] [Google Scholar]
- Olson M., Hood L., Cantor C., Botstein D. A common language for physical mapping of the human genome. Science. 1989 Sep 29;245(4925):1434–1435. doi: 10.1126/science.2781285. [DOI] [PubMed] [Google Scholar]
- Orita M., Sekiya T., Hayashi K. DNA sequence polymorphisms in Alu repeats. Genomics. 1990 Oct;8(2):271–278. doi: 10.1016/0888-7543(90)90282-y. [DOI] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Poduslo S. E., Dean M., Kolch U., O'Brien S. J. Detecting high-resolution polymorphisms in human coding loci by combining PCR and single-strand conformation polymorphism (SSCP) analysis. Am J Hum Genet. 1991 Jul;49(1):106–111. [PMC free article] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rommens J. M., Iannuzzi M. C., Kerem B., Drumm M. L., Melmer G., Dean M., Rozmahel R., Cole J. L., Kennedy D., Hidaka N. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989 Sep 8;245(4922):1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- Sheffield V. C., Cox D. R., Lerman L. S., Myers R. M. Attachment of a 40-base-pair G + C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield V. C., Fishman G. A., Beck J. S., Kimura A. E., Stone E. M. Identification of novel rhodopsin mutations associated with retinitis pigmentosa by GC-clamped denaturing gradient gel electrophoresis. Am J Hum Genet. 1991 Oct;49(4):699–706. [PMC free article] [PubMed] [Google Scholar]
- Stinissen P., Vandenberghe A., Van Broeckhoven C. PCR detection of two RFLP's at the D21S13 locus. Nucleic Acids Res. 1990 Jun 25;18(12):3672–3672. doi: 10.1093/nar/18.12.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theophilus B. D., Latham T., Grabowski G. A., Smith F. I. Comparison of RNase A, a chemical cleavage and GC-clamped denaturing gradient gel electrophoresis for the detection of mutations in exon 9 of the human acid beta-glucosidase gene. Nucleic Acids Res. 1989 Oct 11;17(19):7707–7722. doi: 10.1093/nar/17.19.7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traystman M. D., Higuchi M., Kasper C. K., Antonarakis S. E., Kazazian H. H., Jr Use of denaturing gradient gel electrophoresis to detect point mutations in the factor VIII gene. Genomics. 1990 Feb;6(2):293–301. doi: 10.1016/0888-7543(90)90569-g. [DOI] [PubMed] [Google Scholar]
- Tsuzuki T., Mita S., Maeda S., Araki S., Shimada K. Structure of the human prealbumin gene. J Biol Chem. 1985 Oct 5;260(22):12224–12227. [PubMed] [Google Scholar]
- Weber J. L. Informativeness of human (dC-dA)n.(dG-dT)n polymorphisms. Genomics. 1990 Aug;7(4):524–530. doi: 10.1016/0888-7543(90)90195-z. [DOI] [PubMed] [Google Scholar]
- Yamaki K., Tsuda M., Kikuchi T., Chen K. H., Huang K. P., Shinohara T. Structural organization of the human S-antigen gene. cDNA, amino acid, intron, exon, promoter, in vitro transcription, retina, and pineal gland. J Biol Chem. 1990 Dec 5;265(34):20757–20762. [PubMed] [Google Scholar]
- Yamaki K., Tsuda M., Shinohara T. The sequence of human retinal S-antigen reveals similarities with alpha-transducin. FEBS Lett. 1988 Jul 4;234(1):39–43. doi: 10.1016/0014-5793(88)81298-5. [DOI] [PubMed] [Google Scholar]