Abstract
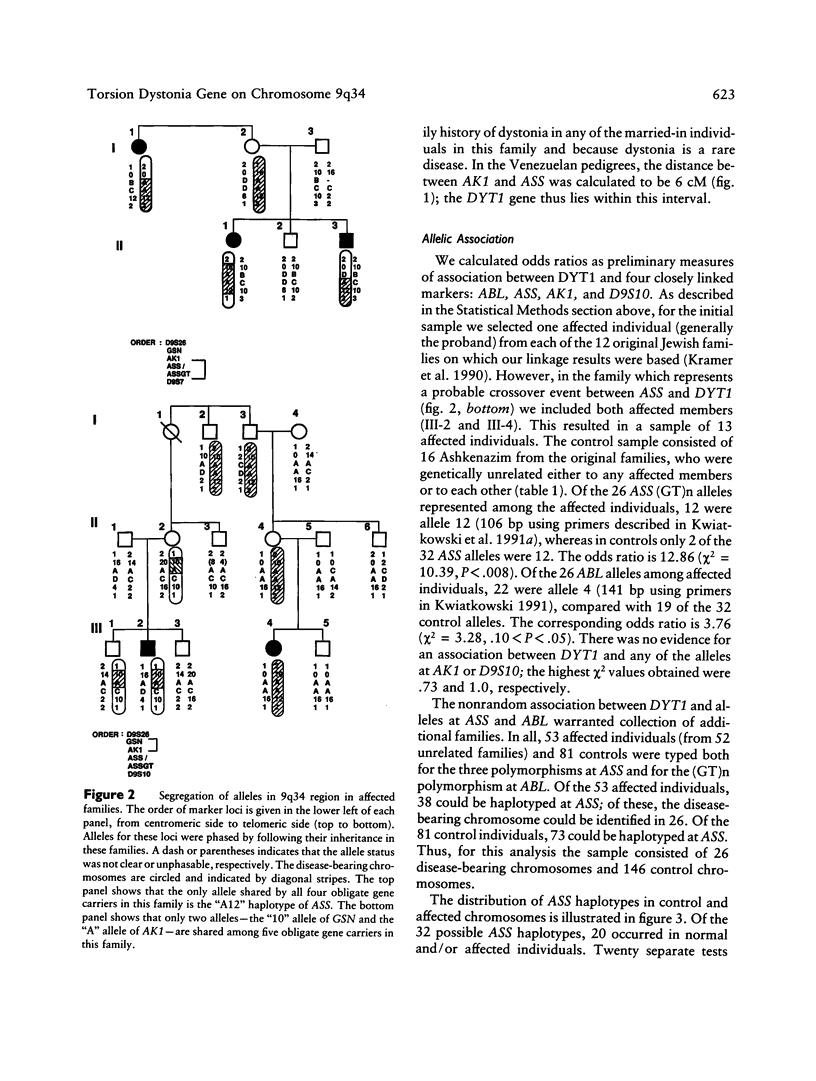
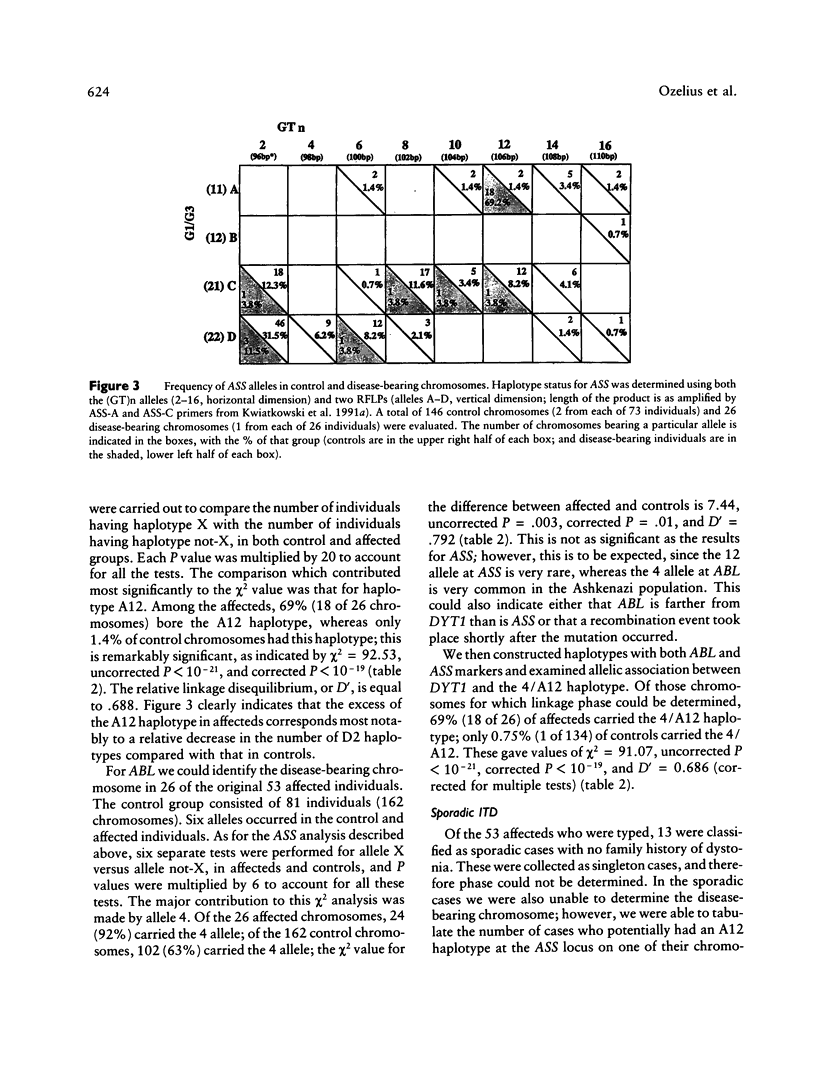
The DYT1 gene responsible for early-onset, idiopathic torsion dystonia (ITD) in the Ashkenazi Jewish population, as well as in one large non-Jewish family, has been mapped to chromosome 9q32-34. Using (GT)n and RFLP markers in this region, we have identified obligate recombination events in some of these Jewish families, which further delineate the area containing the DYT1 gene to a 6-cM region bounded by loci AK1 and ASS. In 52 unrelated, affected Ashkenazi Jewish individuals, we have found highly significant linkage disequilibrium between a particular extended haplotype at the ABL-ASS loci and the DYT1 gene. The 4/A12 haplotype for ABL-ASS is present on 69% of the disease-bearing chromosomes among affected Jewish individuals and on only 1% of control Jewish chromosomes (χ2 = 91.07, P « .001). The allelic association between this extended haplotype and DYT1 predicts that these three genes lie within 1–2 cM of each other; on the basis of obligate recombination events, the DYT1 gene is centromeric to ASS. Furthermore, this allelic association supports the idea that a single mutation event is responsible for most hereditary cases of dystonia in the Jewish population. Of the 53 definitely affecteds typed, 13 appear to be sporadic, with no family history of dystonia. However, the proportion of sporadic cases which potentially carry the A12 haplotype at ASS (8/13 [62%]) is similar to the proportion of familial cases with A12 (28/40 [70%]). This suggests that many sporadic cases are hereditary, that the disease gene frequency is greater than 1/15,000, and that the penetrance is lower than 30%, as previously estimated in this population. Most affected individuals were heterozygous for the ABL-ASS haplotype, a finding supporting autosomal dominant inheritance of the DYT1 gene. The ABL-ASS extended-haplotype status will provide predictive value for carrier status in Jewish individuals. This information can be used for molecular diagnosis, evaluation of subclinical expression of the disease, and elucidation of environmental factors which may modify clinical symptoms.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. A., Gusella J. F. Use of cyclosporin A in establishing Epstein-Barr virus-transformed human lymphoblastoid cell lines. In Vitro. 1984 Nov;20(11):856–858. doi: 10.1007/BF02619631. [DOI] [PubMed] [Google Scholar]
- Axelrod F. B., Pearson J. Congenital sensory neuropathies. Diagnostic distinction from familial dysautonomia. Am J Dis Child. 1984 Oct;138(10):947–954. [PubMed] [Google Scholar]
- Bech-Hansen N. T., Marshall K. J., Kraus S. L. TaqI RFLP in human adenylate kinase-1 (AK1) gene region on chromosome 9. Nucleic Acids Res. 1989 May 25;17(10):4004–4004. doi: 10.1093/nar/17.10.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakefield X. O., Bressman S. B., Kramer P. L., Ozelius L., Moskowitz C., Tanzi R., Brin M. F., Hobbs W., Kaufman D., Tobin A. Linkage analysis in a family with dominantly inherited torsion dystonia: exclusion of the pro-opiomelanocortin and glutamic acid decarboxylase genes and other chromosomal regions using DNA polymorphisms. J Neurogenet. 1986 May;3(3):159–175. doi: 10.3109/01677068609106846. [DOI] [PubMed] [Google Scholar]
- Bressman S. B., de Leon D., Brin M. F., Risch N., Burke R. E., Greene P. E., Shale H., Fahn S. Idiopathic dystonia among Ashkenazi Jews: evidence for autosomal dominant inheritance. Ann Neurol. 1989 Nov;26(5):612–620. doi: 10.1002/ana.410260505. [DOI] [PubMed] [Google Scholar]
- Chakravarti A., Buetow K. H., Antonarakis S. E., Waber P. G., Boehm C. D., Kazazian H. H. Nonuniform recombination within the human beta-globin gene cluster. Am J Hum Genet. 1984 Nov;36(6):1239–1258. [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Green P., Helms C., Cartinhour S., Weiffenbach B., Stephens K., Keith T. P., Bowden D. W., Smith D. R., Lander E. S. A genetic linkage map of the human genome. Cell. 1987 Oct 23;51(2):319–337. doi: 10.1016/0092-8674(87)90158-9. [DOI] [PubMed] [Google Scholar]
- Eldridge R. The torsion dystonias: literature review and genetic and clinical studies. Neurology. 1970 Nov;20(11):1–78. doi: 10.1212/wnl.20.11_part_2.1. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fletcher N. A., Harding A. E., Marsden C. D. A genetic study of idiopathic torsion dystonia in the United Kingdom. Brain. 1990 Apr;113(Pt 2):379–395. doi: 10.1093/brain/113.2.379. [DOI] [PubMed] [Google Scholar]
- Fujita R., Hanauer A., Sirugo G., Heilig R., Mandel J. L. Additional polymorphisms at marker loci D9S5 and D9S15 generate extended haplotypes in linkage disequilibrium with Friedreich ataxia. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1796–1800. doi: 10.1073/pnas.87.5.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusella J., Varsanyi-Breiner A., Kao F. T., Jones C., Puck T. T., Keys C., Orkin S., Housman D. Precise localization of human beta-globin gene complex on chromosome 11. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5239–5242. doi: 10.1073/pnas.76.10.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines J. L., Ozelius L. J., McFarlane H., Menon A., Tzall S., Martiniuk F., Hirschhorn R., Gusella J. F. A genetic linkage map of chromosome 17. Genomics. 1990 Sep;8(1):1–6. doi: 10.1016/0888-7543(90)90218-j. [DOI] [PubMed] [Google Scholar]
- Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., Tsui L. C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989 Sep 8;245(4922):1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Kramer P. L., de Leon D., Ozelius L., Risch N., Bressman S. B., Brin M. F., Schuback D. E., Burke R. E., Kwiatkowski D. J., Shale H. Dystonia gene in Ashkenazi Jewish population is located on chromosome 9q32-34. Ann Neurol. 1990 Feb;27(2):114–120. doi: 10.1002/ana.410270203. [DOI] [PubMed] [Google Scholar]
- Kupke K. G., Lee L. V., Viterbo G. H., Arancillo J., Donlon T., Müller U. X-linked recessive torsion dystonia in the Philippines. Am J Med Genet. 1990 Jun;36(2):237–242. doi: 10.1002/ajmg.1320360219. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D. J., Nygaard T. G., Schuback D. E., Perman S., Trugman J. M., Bressman S. B., Burke R. E., Brin M. F., Ozelius L., Breakefield X. O. Identification of a highly polymorphic microsatellite VNTR within the argininosuccinate synthetase locus: exclusion of the dystonia gene on 9q32-34 as the cause of dopa-responsive dystonia in a large kindred. Am J Hum Genet. 1991 Jan;48(1):121–128. [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski D. J., Ozelius L., Kramer P. L., Perman S., Schuback D. E., Gusella J. F., Fahn S., Breakefield X. O. Torsion dystonia genes in two populations confined to a small region on chromosome 9q32-34. Am J Hum Genet. 1991 Aug;49(2):366–371. [PMC free article] [PubMed] [Google Scholar]
- Lander E. S., Green P., Abrahamson J., Barlow A., Daly M. J., Lincoln S. E., Newberg L. A., Newburg L. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987 Oct;1(2):174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- Lathrop M., Nakamura Y., O'Connell P., Leppert M., Woodward S., Lalouel J. M., White R. A mapped set of genetic markers for human chromosome 9. Genomics. 1988 Nov;3(4):361–366. doi: 10.1016/0888-7543(88)90128-0. [DOI] [PubMed] [Google Scholar]
- Lee L. V., Pascasio F. M., Fuentes F. D., Viterbo G. H. Torsion dystonia in Panay, Philippines. Adv Neurol. 1976;14:137–151. [PubMed] [Google Scholar]
- Litt M., Luty J. A. A hypervariable microsatellite revealed by in vitro amplification of a dinucleotide repeat within the cardiac muscle actin gene. Am J Hum Genet. 1989 Mar;44(3):397–401. [PMC free article] [PubMed] [Google Scholar]
- Maayan C., Kaplan E., Shachar S., Peleg O., Godfrey S. Incidence of familial dysautonomia in Israel 1977-1981. Clin Genet. 1987 Aug;32(2):106–108. doi: 10.1111/j.1399-0004.1987.tb03334.x. [DOI] [PubMed] [Google Scholar]
- Mattila P., Korpela J., Tenkanen T., Pitkänen K. Fidelity of DNA synthesis by the Thermococcus litoralis DNA polymerase--an extremely heat stable enzyme with proofreading activity. Nucleic Acids Res. 1991 Sep 25;19(18):4967–4973. doi: 10.1093/nar/19.18.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila P., Korpela J., Tenkanen T., Pitkänen K. Fidelity of DNA synthesis by the Thermococcus litoralis DNA polymerase--an extremely heat stable enzyme with proofreading activity. Nucleic Acids Res. 1991 Sep 25;19(18):4967–4973. doi: 10.1093/nar/19.18.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U., Kupke K. G. The genetics of primary torsion dystonia. Hum Genet. 1990 Jan;84(2):107–115. doi: 10.1007/BF00208922. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Fujimoto E., O'Connell P., Leppert M., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence pEFD126.3 on chromosome 9q (D9S7). Nucleic Acids Res. 1987 Dec 23;15(24):10607–10607. doi: 10.1093/nar/15.24.10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrup H., Lathrop M., Lu S. Y., Daiger S. P., Beaudet A. L., O'Brien W. E. Multilocus linkage analysis with the human argininosuccinate synthetase gene. Genomics. 1989 Oct;5(3):442–444. doi: 10.1016/0888-7543(89)90007-4. [DOI] [PubMed] [Google Scholar]
- Ozelius L., Kramer P. L., Moskowitz C. B., Kwiatkowski D. J., Brin M. F., Bressman S. B., Schuback D. E., Falk C. T., Risch N., de Leon D. Human gene for torsion dystonia located on chromosome 9q32-q34. Neuron. 1989 May;2(5):1427–1434. doi: 10.1016/0896-6273(89)90188-8. [DOI] [PubMed] [Google Scholar]
- Pauls D. L., Korczyn A. D. Complex segregation analysis of dystonia pedigrees suggests autosomal dominant inheritance. Neurology. 1990 Jul;40(7):1107–1110. doi: 10.1212/wnl.40.7.1107. [DOI] [PubMed] [Google Scholar]
- Risch N. J., Bressman S. B., deLeon D., Brin M. F., Burke R. E., Greene P. E., Shale H., Claus E. B., Cupples L. A., Fahn S. Segregation analysis of idiopathic torsion dystonia in Ashkenazi Jews suggests autosomal dominant inheritance. Am J Hum Genet. 1990 Mar;46(3):533–538. [PMC free article] [PubMed] [Google Scholar]
- Schuback D. E., Ozelius L., Breakefield X. O. BanI RFLP at AK1 locus (9q34). Nucleic Acids Res. 1991 Oct 25;19(20):5798–5798. doi: 10.1093/nar/19.20.5798-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson G. A review of theoretical aspects of HLA and disease associations. Theor Popul Biol. 1981 Oct;20(2):168–208. doi: 10.1016/0040-5809(81)90009-5. [DOI] [PubMed] [Google Scholar]
- Trofatter J. A., Haines J. L., Conneally P. M. LIPIN: an interactive data entry and management program for LIPED. Am J Hum Genet. 1986 Jul;39(1):147–148. [PMC free article] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- Zeman W., Dyken P. Dystonia musculorum deformans. Clinical, genetic and pathoanatomical studies. Psychiatr Neurol Neurochir. 1967 Mar-Apr;70(2):77–121. [PubMed] [Google Scholar]
- Zilber N., Korczyn A. D., Kahana E., Fried K., Alter M. Inheritance of idiopathic torsion dystonia among Jews. J Med Genet. 1984 Feb;21(1):13–20. doi: 10.1136/jmg.21.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]


