Abstract
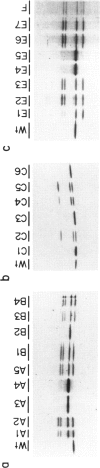
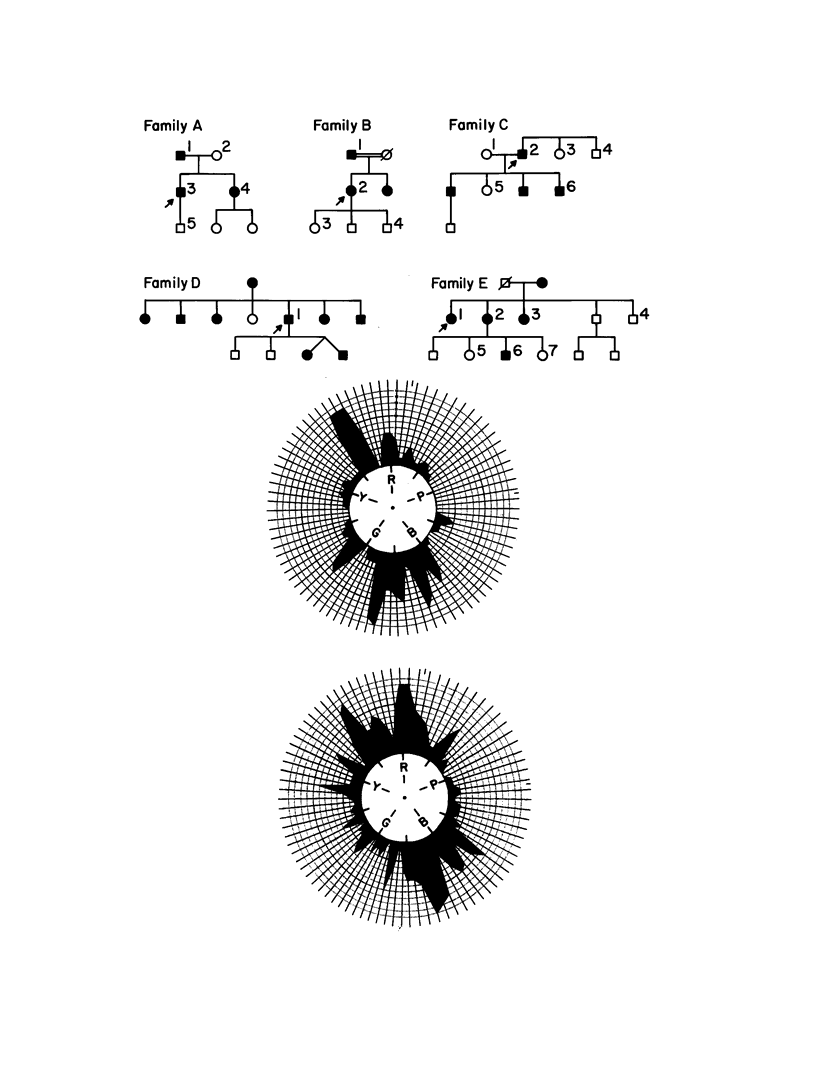
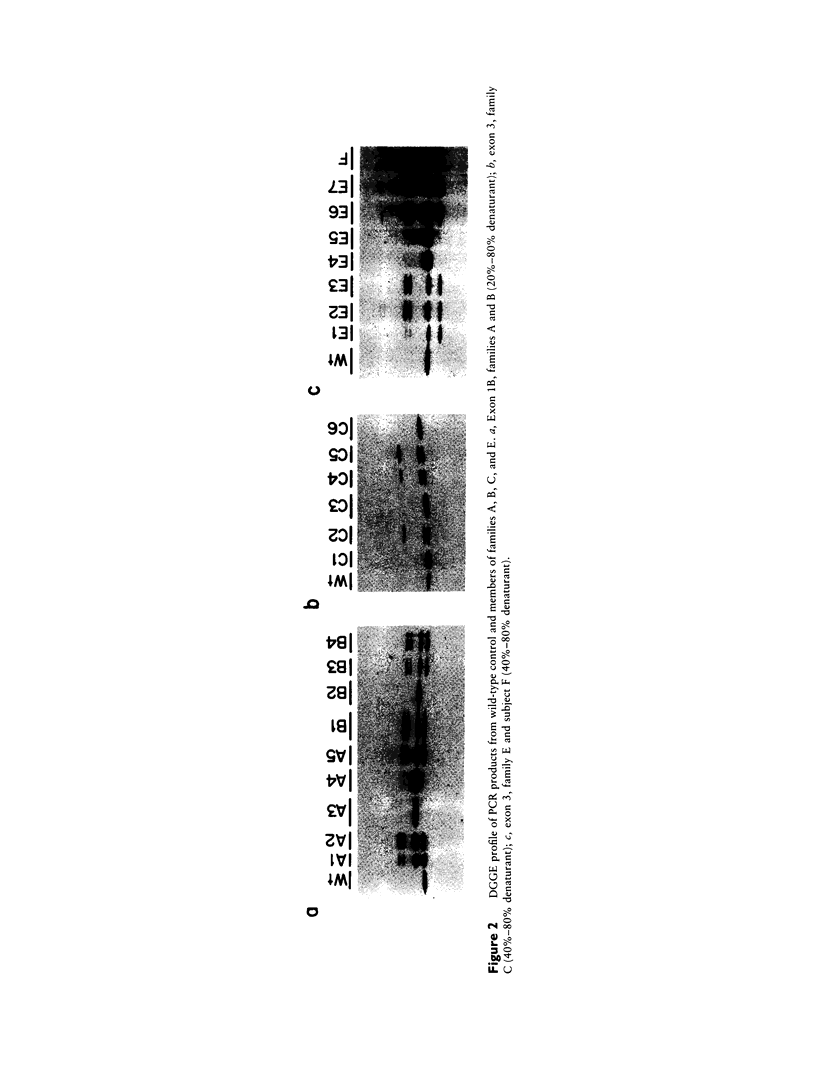
Tritanopia is an autosomal dominant genetic disorder of human vision characterize by a selective deficiency of blue spectral sensitivity. The defect is manifested within the retina and could be caused by a deficiency in function or numbers (or both) of blue-sensitive cone photoreceptors. We have used PCR, denaturing gradient gel electrophoresis, and DNA sequencing of amplified exons to detect in four of nine unrelated tritanopic subjects two different point mutations in the gene encoding the blue-sensitive opsin, each leading to an amino acid substitution. Segregation analysis within pedigrees and hybridization of oligonucleotides specific for each allele to DNA samples from control subjects support the hypothesis that these mutations cause tritanopia. These results complete the genetic evidence for the trichromatic theory of human color vision.
Full text
PDF



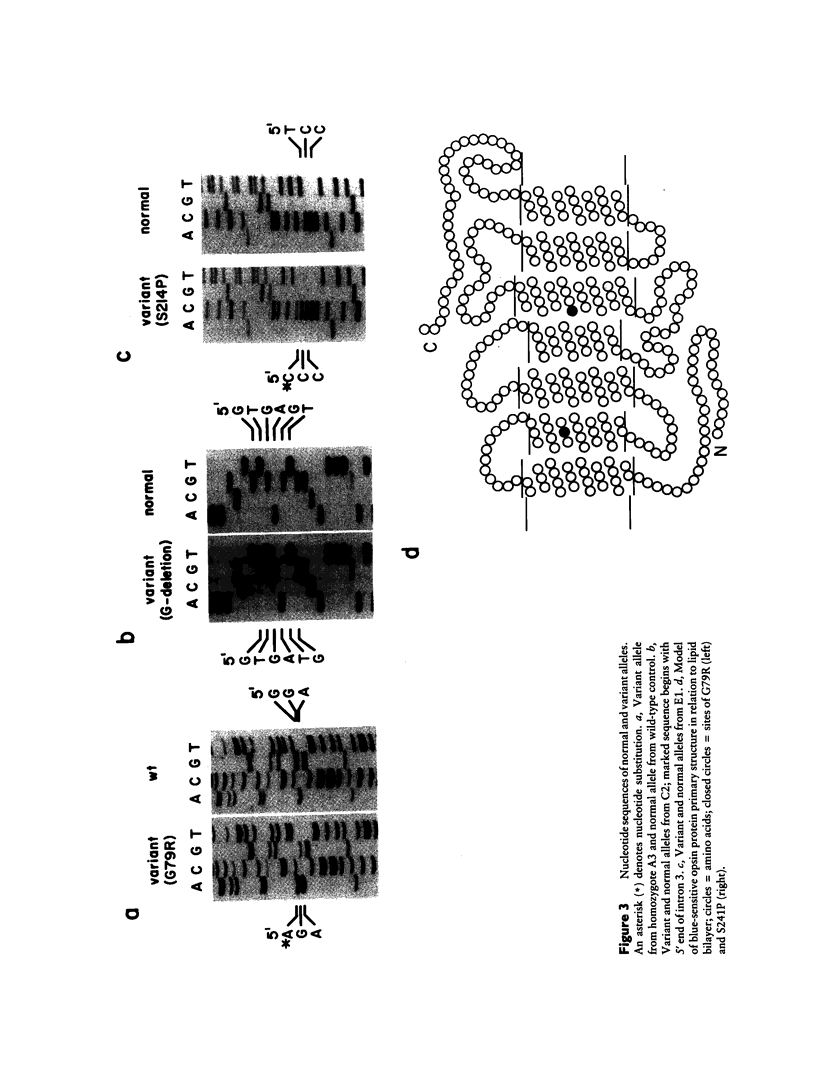
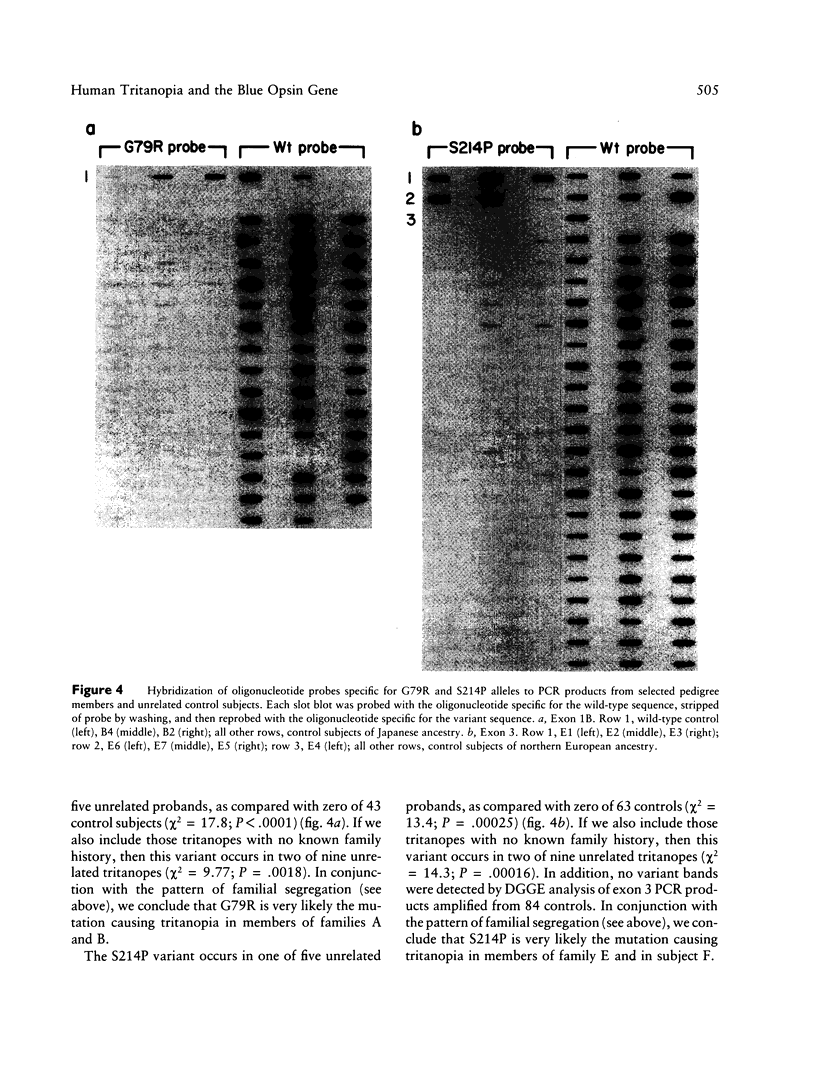


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dryja T. P., McGee T. L., Hahn L. B., Cowley G. S., Olsson J. E., Reichel E., Sandberg M. A., Berson E. L. Mutations within the rhodopsin gene in patients with autosomal dominant retinitis pigmentosa. N Engl J Med. 1990 Nov 8;323(19):1302–1307. doi: 10.1056/NEJM199011083231903. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., McGee T. L., Reichel E., Hahn L. B., Cowley G. S., Yandell D. W., Sandberg M. A., Berson E. L. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990 Jan 25;343(6256):364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- Fischer S. G., Lerman L. S. DNA fragments differing by single base-pair substitutions are separated in denaturing gradient gels: correspondence with melting theory. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1579–1583. doi: 10.1073/pnas.80.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins K. E., Brooks D. N., Gottschalk G. Tritan pedigree without optic-nerve atrophy. Am J Optom Physiol Opt. 1983 Dec;60(12):964–969. doi: 10.1097/00006324-198312000-00004. [DOI] [PubMed] [Google Scholar]
- KALMUS H. The familial distribution of congenital tritanopia, with some remarks on some similar conditions. Ann Hum Genet. 1955 Aug;20(1):39–56. doi: 10.1111/j.1469-1809.1955.tb01277.x. [DOI] [PubMed] [Google Scholar]
- Miyake Y., Yagasaki K., Ichikawa H. Differential diagnosis of congenital tritanopia and dominantly inherited juvenile optic atrophy. Arch Ophthalmol. 1985 Oct;103(10):1496–1501. doi: 10.1001/archopht.1985.01050100072022. [DOI] [PubMed] [Google Scholar]
- Nagamine C. M., Chan K., Lau Y. F. A PCR artifact: generation of heteroduplexes. Am J Hum Genet. 1989 Aug;45(2):337–339. [PMC free article] [PubMed] [Google Scholar]
- Nathans J., Piantanida T. P., Eddy R. L., Shows T. B., Hogness D. S. Molecular genetics of inherited variation in human color vision. Science. 1986 Apr 11;232(4747):203–210. doi: 10.1126/science.3485310. [DOI] [PubMed] [Google Scholar]
- Nathans J., Thomas D., Hogness D. S. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986 Apr 11;232(4747):193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- Neuhann T., Kalmus H., Jaeger W. Ophthalmological findings in the tritans, described by Wright and Kalmus. Mod Probl Ophthalmol. 1976;17:135–142. [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Padmos P., van Norren D., Faijer J. W. Blue cone function in a family with an inherited tritan defect, tested with electroretinography and psychophysics. Invest Ophthalmol Vis Sci. 1978 May;17(5):436–441. [PubMed] [Google Scholar]
- Pakula A. A., Sauer R. T. Genetic analysis of protein stability and function. Annu Rev Genet. 1989;23:289–310. doi: 10.1146/annurev.ge.23.120189.001445. [DOI] [PubMed] [Google Scholar]
- Pokorny J., Smith V. C., Went L. N. Color matching in autosomal dominant tritan defect. J Opt Soc Am. 1981 Nov;71(11):1327–1334. doi: 10.1364/josa.71.001327. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sakmar T. P., Franke R. R., Khorana H. G. Glutamic acid-113 serves as the retinylidene Schiff base counterion in bovine rhodopsin. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8309–8313. doi: 10.1073/pnas.86.21.8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz G. E., Elzinga M., Marx F., Schrimer R. H. Three dimensional structure of adenyl kinase. Nature. 1974 Jul 12;250(462):120–123. doi: 10.1038/250120a0. [DOI] [PubMed] [Google Scholar]
- Sheffield V. C., Cox D. R., Lerman L. S., Myers R. M. Attachment of a 40-base-pair G + C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung C. H., Davenport C. M., Hennessey J. C., Maumenee I. H., Jacobson S. G., Heckenlively J. R., Nowakowski R., Fishman G., Gouras P., Nathans J. Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6481–6485. doi: 10.1073/pnas.88.15.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Orkin S. H., Maniatis T. Specific transcription and RNA splicing defects in five cloned beta-thalassaemia genes. Nature. 1983 Apr 14;302(5909):591–596. doi: 10.1038/302591a0. [DOI] [PubMed] [Google Scholar]
- WRIGHT W. D. The characteristics of tritanopia. J Opt Soc Am. 1952 Aug;42(8):509–521. doi: 10.1364/josa.42.000509. [DOI] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- Went L. N., Pronk N. The genetics of tritan disturbances. Hum Genet. 1985;69(3):255–262. doi: 10.1007/BF00293036. [DOI] [PubMed] [Google Scholar]
- Wooten B. R., Wald G. Color-vision mechanisms in the peripheral retinas of normal and dichromatic observers. J Gen Physiol. 1973 Feb;61(2):125–145. doi: 10.1085/jgp.61.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]