Abstract
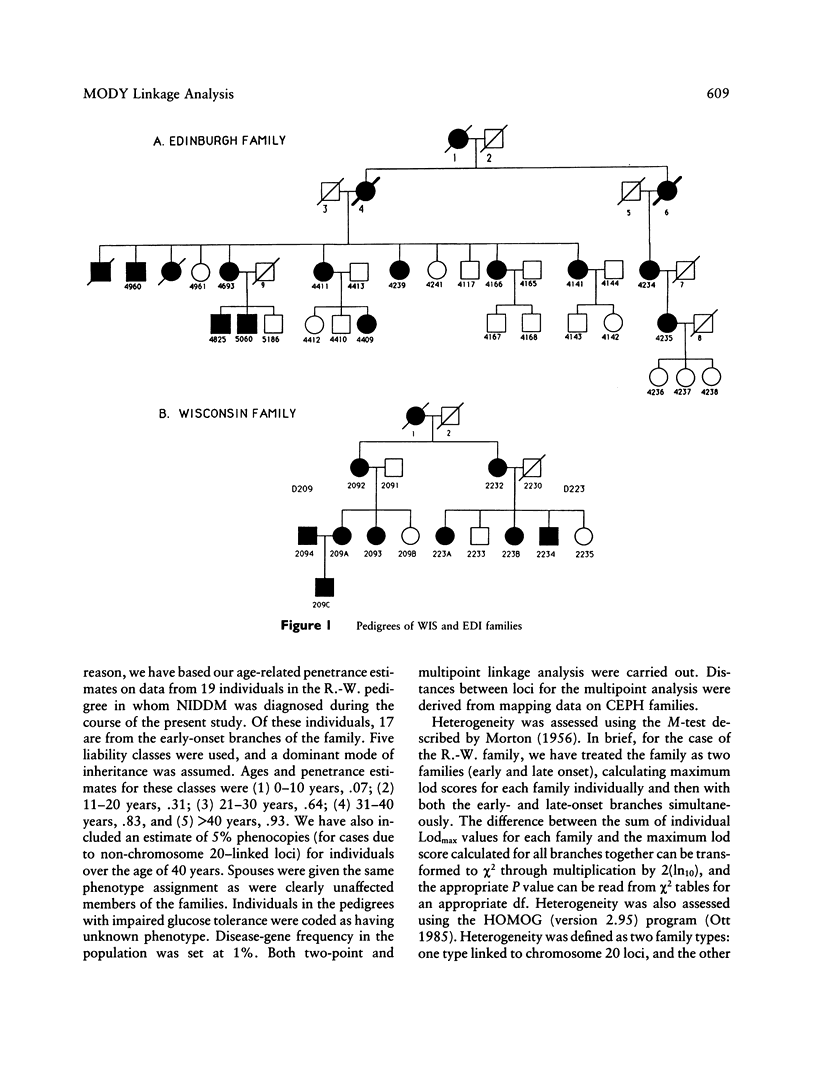
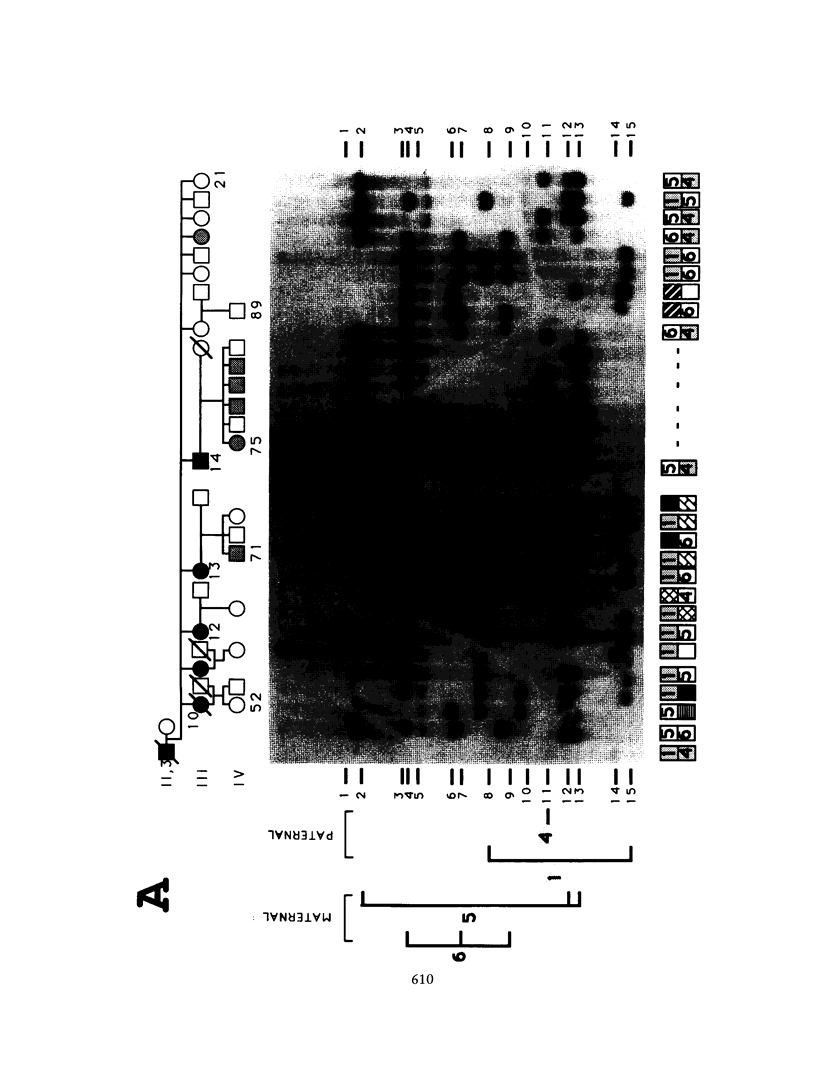
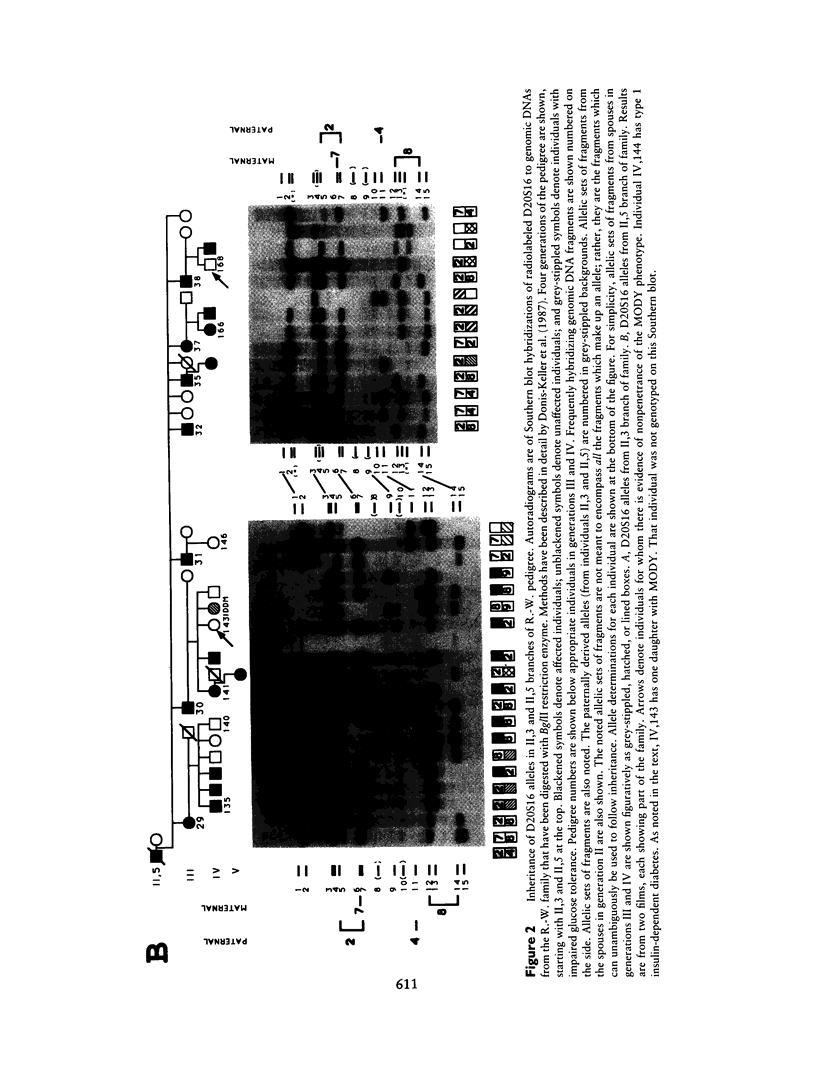
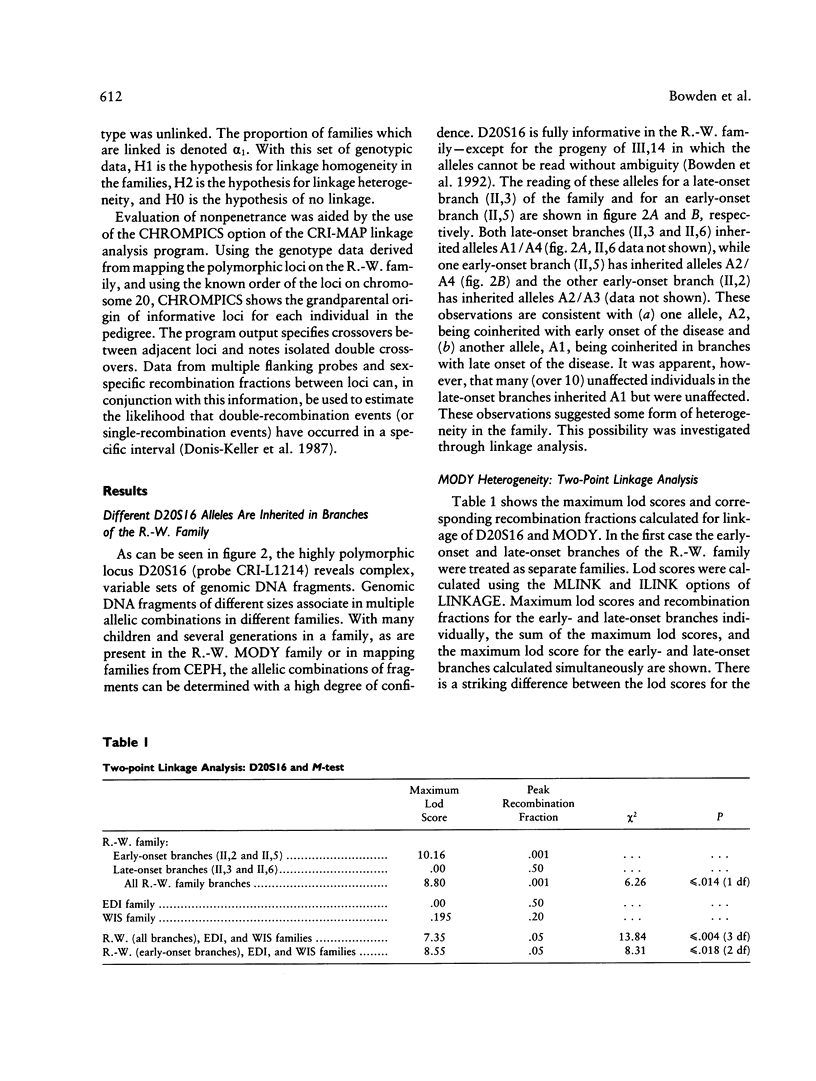
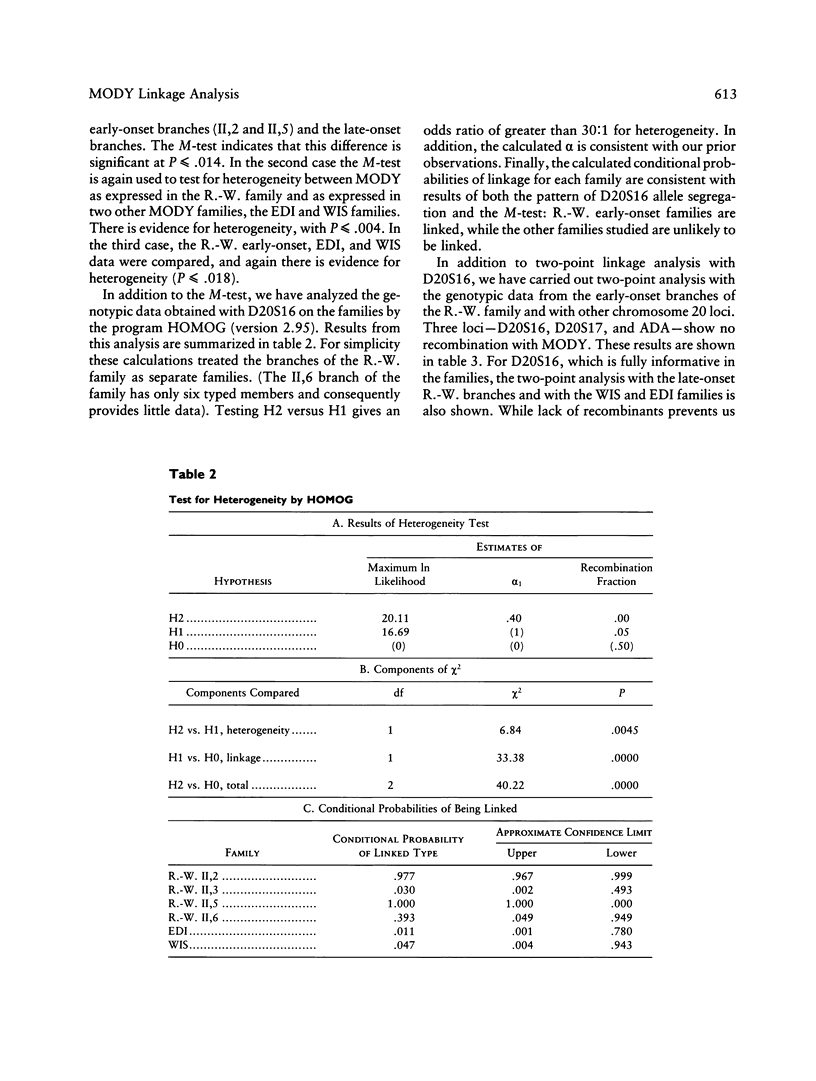
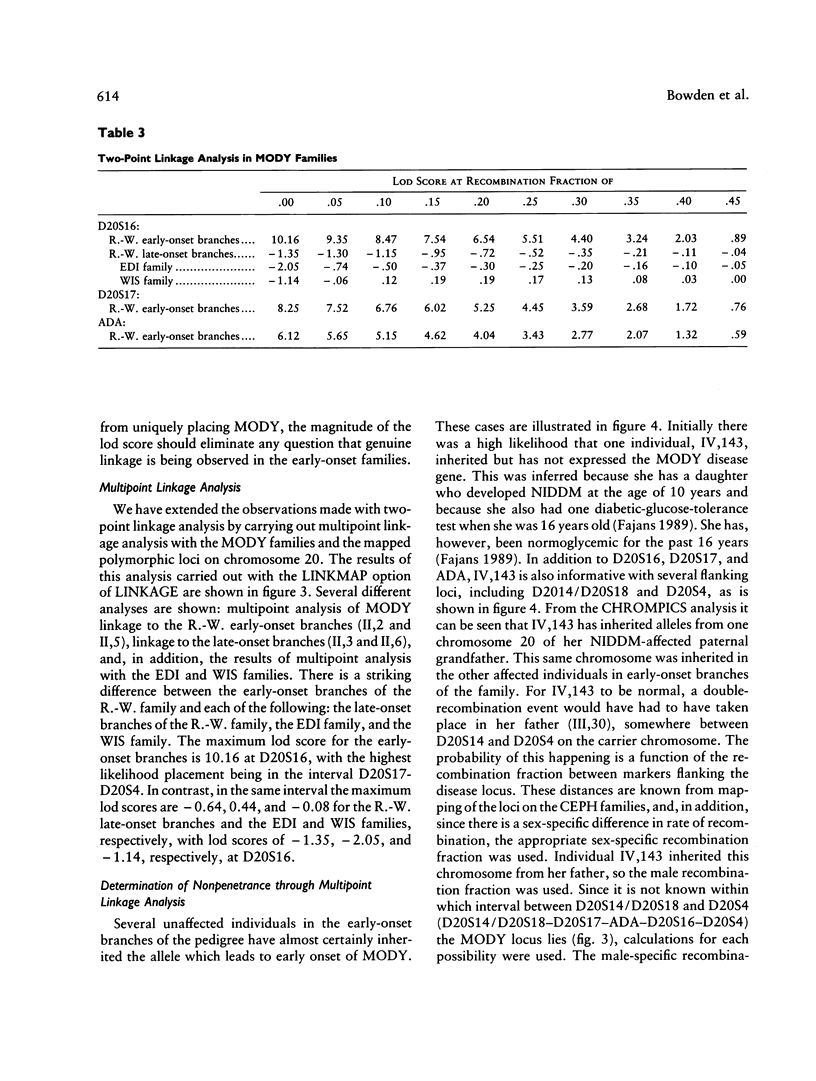
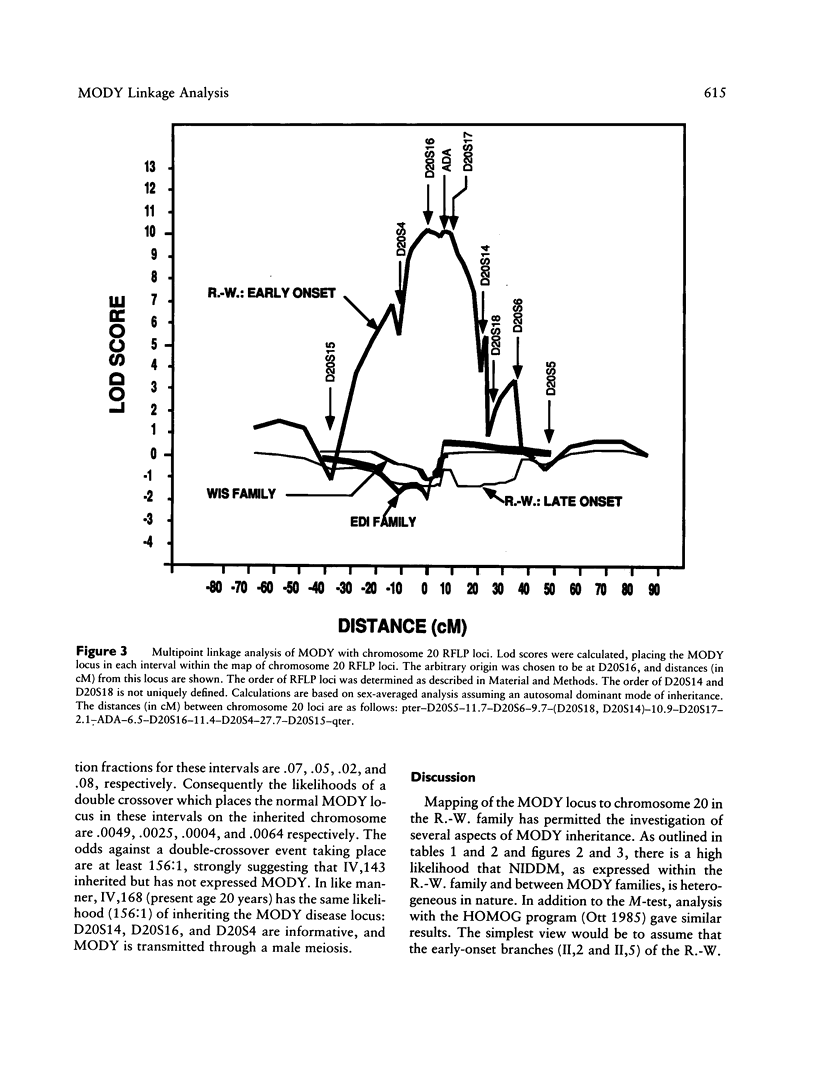
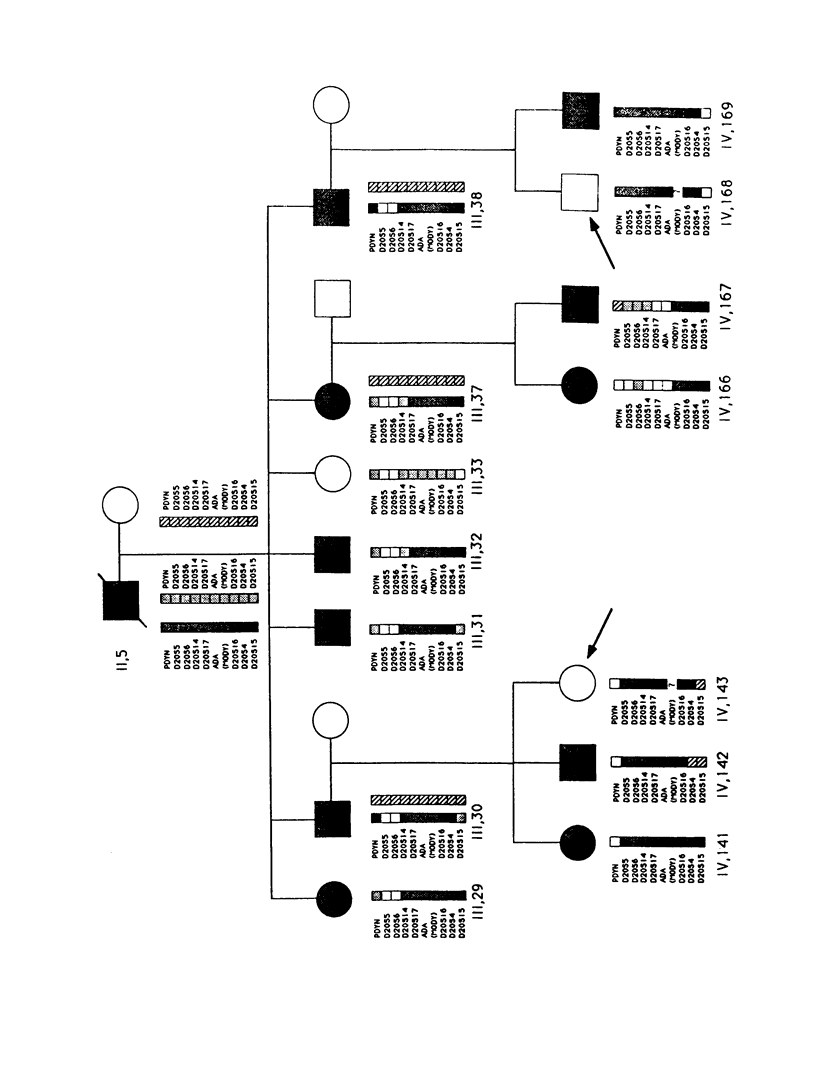
We have analyzed the inheritance of maturity-onset diabetes of the young (MODY) on chromosome 20 in a large multigeneration family, the R.-W. family, and in two other MODY families. Of the four branches of the R.-W. pedigree which have been studied, two have documented early onset of non-insulin-dependent diabetes mellitus (NIDDM), while there is no evidence of early onset in the other two branches. The early-onset branches have apparently inherited the same D20S16 allele from the affected parent, while another D20S16 allele was inherited in the two branches without evidence of early onset. A test for homogeneity, the M-test, using the results of two-point linkage analysis with D20S16 indicates heterogeneity between early- and late-onset branches of the R.-W. family (P ≤ .014). In addition, analysis strongly suggests that MODY as expressed in the EDI and WIS families is unlinked to loci on chromosome 20 (P ≤ .018–.004). Comparable results are seen when the data are analyzed by the HOMOG program. Three polymorphic loci–D20S16, D20S17, and ADA–show no recombination with the MODY locus when two-point linkage analysis is used in the early-onset branches of the family. The multipoint lod score in the early-onset branches of the R.-W. family is 10.16, with the most likely location being between D20S4 and D20S17. Multipoint linkage analysis using the CHROMPICS option of the program CRI-MAP has been used to follow inheritance of the MODY disease locus. This analysis has identified two cases of possible nonpenetrance in the early-onset branches of the family (odds of at least 156:1), as determined by the appearance of apparent isolated double crossovers at the MODY locus in these unaffected individuals.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett A. H., Eff C., Leslie R. D., Pyke D. A. Diabetes in identical twins. A study of 200 pairs. Diabetologia. 1981 Feb;20(2):87–93. doi: 10.1007/BF00262007. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Xiang K. S., Newman M. V., Wu S. H., Wright L. G., Fajans S. S., Spielman R. S., Cox N. J. Gene for non-insulin-dependent diabetes mellitus (maturity-onset diabetes of the young subtype) is linked to DNA polymorphism on human chromosome 20q. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1484–1488. doi: 10.1073/pnas.88.4.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden D. W., Gravius T. C., Akots G., Fajans S. S. Identification of genetic markers flanking the locus for maturity-onset diabetes of the young on human chromosome 20. Diabetes. 1992 Jan;41(1):88–92. doi: 10.2337/diab.41.1.88. [DOI] [PubMed] [Google Scholar]
- Bowden D. W., Gravius T. C., Green P., Falls K., Wurster-Hill D., Noll W., Müller-Kahle H., Donis-Keller H. A genetic linkage map of 32 loci on human chromosome 10. Genomics. 1989 Nov;5(4):718–726. doi: 10.1016/0888-7543(89)90113-4. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Green P., Helms C., Cartinhour S., Weiffenbach B., Stephens K., Keith T. P., Bowden D. W., Smith D. R., Lander E. S. A genetic linkage map of the human genome. Cell. 1987 Oct 23;51(2):319–337. doi: 10.1016/0092-8674(87)90158-9. [DOI] [PubMed] [Google Scholar]
- Economou E. P., Bergen A. W., Warren A. C., Antonarakis S. E. The polydeoxyadenylate tract of Alu repetitive elements is polymorphic in the human genome. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2951–2954. doi: 10.1073/pnas.87.8.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajans S. S. MODY--a model for understanding the pathogeneses and natural history of type II diabetes. Horm Metab Res. 1987 Dec;19(12):591–599. doi: 10.1055/s-2007-1011888. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. J., Duncan A. M., Farrer L. A., Holden J. J., White B. N., Kidd J. R., Kidd K. K., Simpson N. E. Localization and linkage of three polymorphic DNA sequences on human chromosome 20. Cytogenet Cell Genet. 1987;44(2-3):112–117. doi: 10.1159/000132354. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON N. E. The detection and estimation of linkage between the genes for elliptocytosis and the Rh blood type. Am J Hum Genet. 1956 Jun;8(2):80–96. [PMC free article] [PubMed] [Google Scholar]
- Schumm J. W., Knowlton R. G., Braman J. C., Barker D. F., Botstein D., Akots G., Brown V. A., Gravius T. C., Helms C., Hsiao K. Identification of more than 500 RFLPs by screening random genomic clones. Am J Hum Genet. 1988 Jan;42(1):143–159. [PMC free article] [PubMed] [Google Scholar]