Abstract
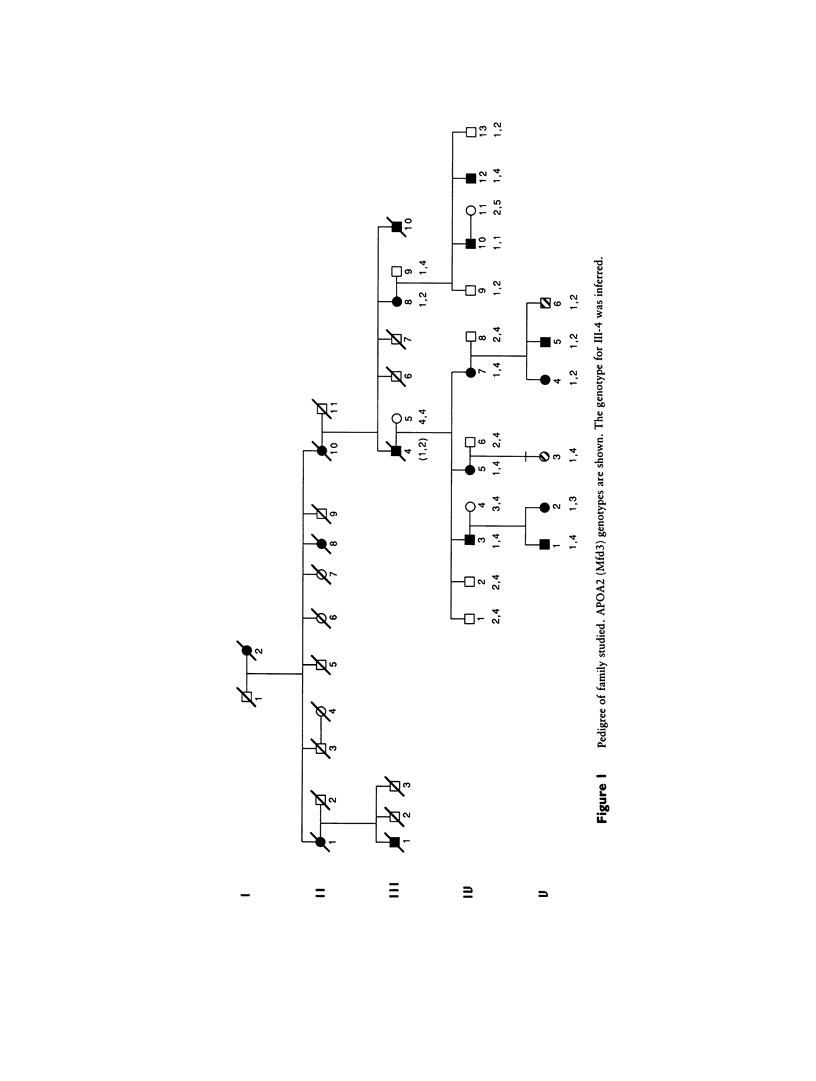
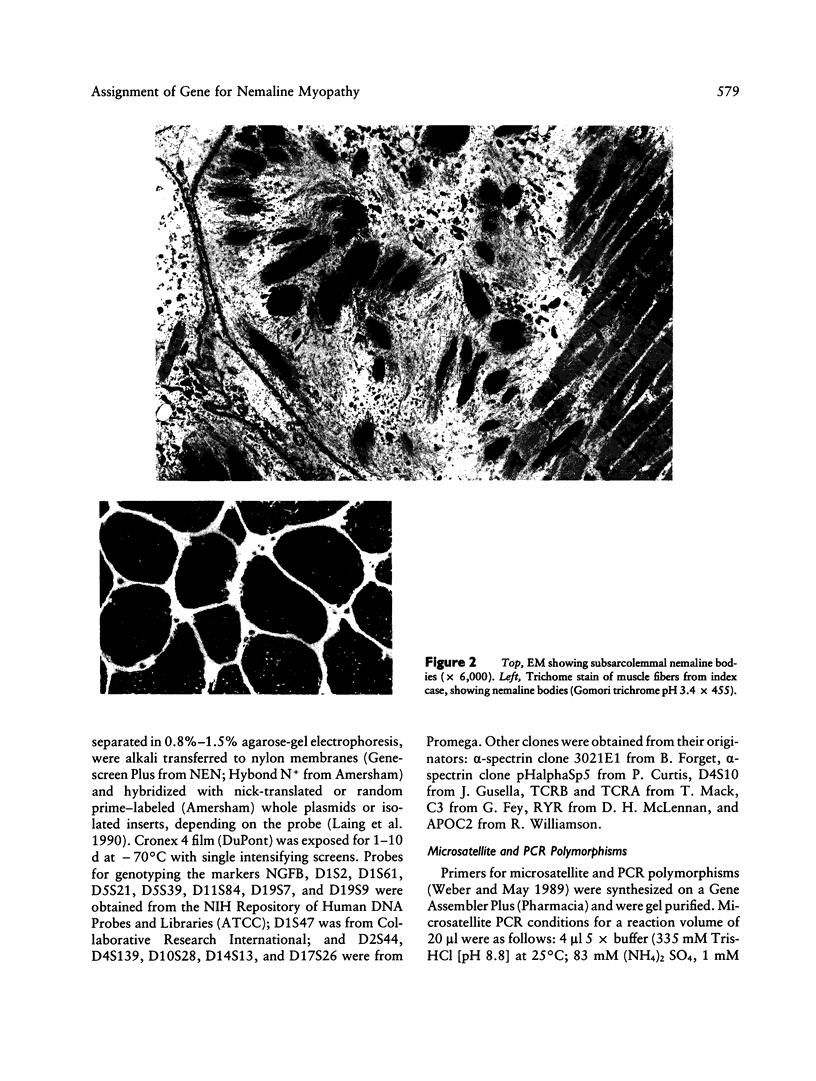
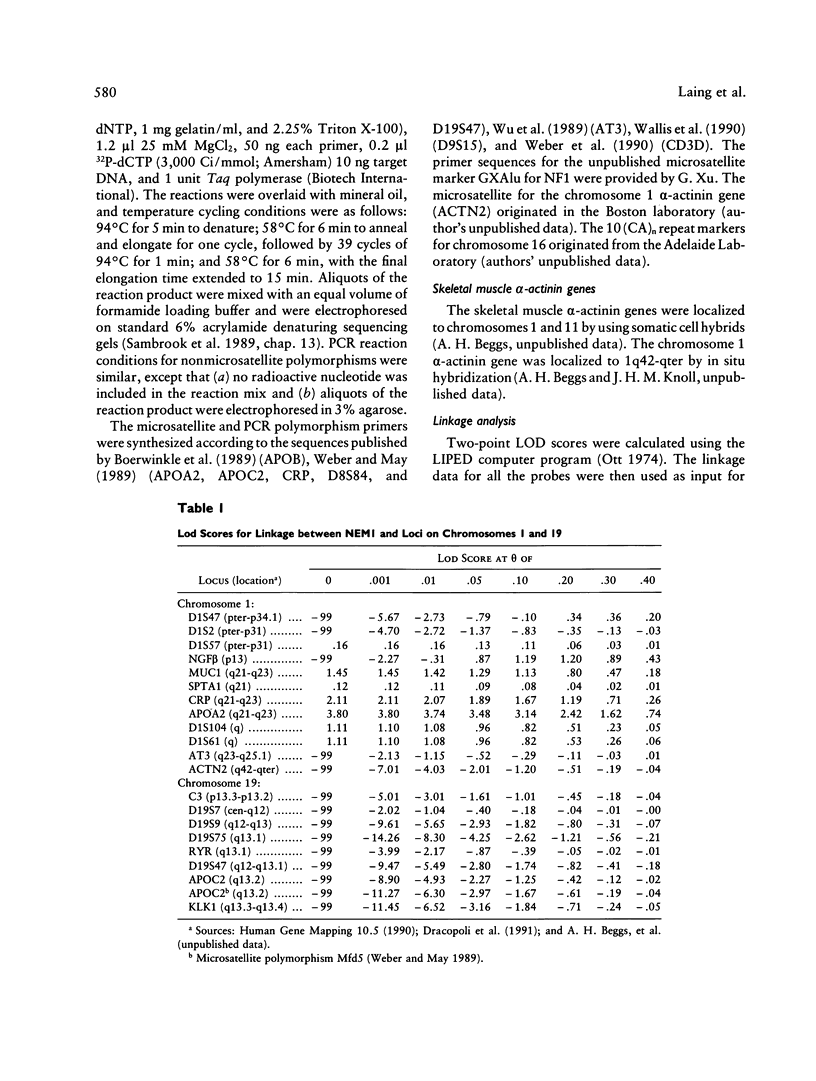
Nemaline myopathy (NEM) is a neuromuscular disorder characterized by the presence, in skeletal muscle, of nemaline rods composed at least in part of α-actinin. A candidate gene and linkage approach was used to localize the gene (NEM1) for an autosomal dominant form (MIM 161800) in one large kindred with 10 living affected family members. Markers on chromosome 19 that were linked to the central core disease gene, a marker at the complement 3 locus, and a marker on chromosome 1 at the α-actinin locus exclude these three candidate genes. The family was fully informative for APOA2, which is localized to 1q21-q23. NEM1 was assigned to chromosome 1 by close linkage for APOA2, which is localized to 1q21-q23. NEM1 was assigned to chromosome 1 by close linkage to APOA2, with a lod score of 3.8 at a recombination fraction of 0. Recombinants with NGFB (1p13) and AT3 (1q23-25.1) indicate that NEM1 lies between 1p13 and 1q25.1. In total, 47 loci were investigated on chromosomes 1, 2, 4, 5, 7–11, 14, 16, 17, and 19, with no indications of significant linkage other than to markers on chromosome 1.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boerwinkle E., Xiong W. J., Fourest E., Chan L. Rapid typing of tandemly repeated hypervariable loci by the polymerase chain reaction: application to the apolipoprotein B 3' hypervariable region. Proc Natl Acad Sci U S A. 1989 Jan;86(1):212–216. doi: 10.1073/pnas.86.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K. E., Jackson J., Williamson R., Harper P. S., Ball S., Sarfarazi M., Meredith L., Fey G. Linkage analysis of myotonic dystrophy and sequences on chromosome 19 using a cloned complement 3 gene probe. J Med Genet. 1983 Aug;20(4):259–263. doi: 10.1136/jmg.20.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dracopoli N. C., O'Connell P., Elsner T. I., Lalouel J. M., White R. L., Buetow K. H., Nishimura D. Y., Murray J. C., Helms C., Mishra S. K. The CEPH consortium linkage map of human chromosome 1. Genomics. 1991 Apr;9(4):686–700. doi: 10.1016/0888-7543(91)90362-i. [DOI] [PubMed] [Google Scholar]
- Edwards J. H. Exclusion mapping. J Med Genet. 1987 Sep;24(9):539–543. doi: 10.1136/jmg.24.9.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan E. A., Freemantle C. J., McCure J. A., Friend K. L., Mulley J. C. Assignment of the gene for central core disease to chromosome 19. Hum Genet. 1990 Dec;86(2):187–190. doi: 10.1007/BF00197703. [DOI] [PubMed] [Google Scholar]
- Jennekens F. G., Roord J. J., Veldman H., Willemse J., Jockusch B. M. Congenital nemaline myopathy. I. Defective organization of alpha-actinin is restricted to muscle. Muscle Nerve. 1983 Jan;6(1):61–68. doi: 10.1002/mus.880060111. [DOI] [PubMed] [Google Scholar]
- Laing N. G., Mears M. E., Thomas H. E., Chandler D. C., Layton M. G., Goldblatt J., Kakulas B. A. Differentiation of Becker muscular dystrophy from limb-girdle muscular dystrophy and Kugelberg-Welander disease using a cDNA probe. Med J Aust. 1990 Mar 5;152(5):270–271. doi: 10.5694/j.1326-5377.1990.tb120926.x. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco A. P., Bertelson C. J., Liechti-Gallati S., Moser H., Kunkel L. M. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988 Jan;2(1):90–95. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Leppert M., O'Connell P., Wolff R., Holm T., Culver M., Martin C., Fujimoto E., Hoff M., Kumlin E. Variable number of tandem repeat (VNTR) markers for human gene mapping. Science. 1987 Mar 27;235(4796):1616–1622. doi: 10.1126/science.3029872. [DOI] [PubMed] [Google Scholar]
- Neitzel H. A routine method for the establishment of permanent growing lymphoblastoid cell lines. Hum Genet. 1986 Aug;73(4):320–326. doi: 10.1007/BF00279094. [DOI] [PubMed] [Google Scholar]
- Ott J. Estimation of the recombination fraction in human pedigrees: efficient computation of the likelihood for human linkage studies. Am J Hum Genet. 1974 Sep;26(5):588–597. [PMC free article] [PubMed] [Google Scholar]
- SHY G. M., ENGEL W. K., SOMERS J. E., WANKO T. NEMALINE MYOPATHY. A NEW CONGENITAL MYOPATHY. Brain. 1963 Dec;86:793–810. doi: 10.1093/brain/86.4.793. [DOI] [PubMed] [Google Scholar]
- Sarfarazi M., Upadhyaya M., Padberg G., Pericak-Vance M., Siddique T., Lucotte G., Lunt P. An exclusion map for facioscapulohumeral (Landouzy-Déjérine) disease. J Med Genet. 1989 Aug;26(8):481–484. doi: 10.1136/jmg.26.8.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance J. M. Hereditary motor and sensory neuropathies. J Med Genet. 1991 Jan;28(1):1–5. doi: 10.1136/jmg.28.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis J., Williamson R., Chamberlain S. Identification of a hypervariable microsatellite polymorphism within D9S15 tightly linked to Friedrich's ataxia. Hum Genet. 1990 Jun;85(1):98–100. doi: 10.1007/BF00276331. [DOI] [PubMed] [Google Scholar]
- Weber J. L., Kwitek A. E., May P. E. Dinucleotide repeat polymorphisms at the D11S419 and CD3D loci. Nucleic Acids Res. 1990 Jul 11;18(13):4036–4036. doi: 10.1093/nar/18.13.4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- Wu S., Seino S., Bell G. I. Human antithrombin II (AT3) gene length polymorphism revealed by the polymerase chain reaction. Nucleic Acids Res. 1989 Aug 11;17(15):6433–6433. doi: 10.1093/nar/17.15.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]