Abstract
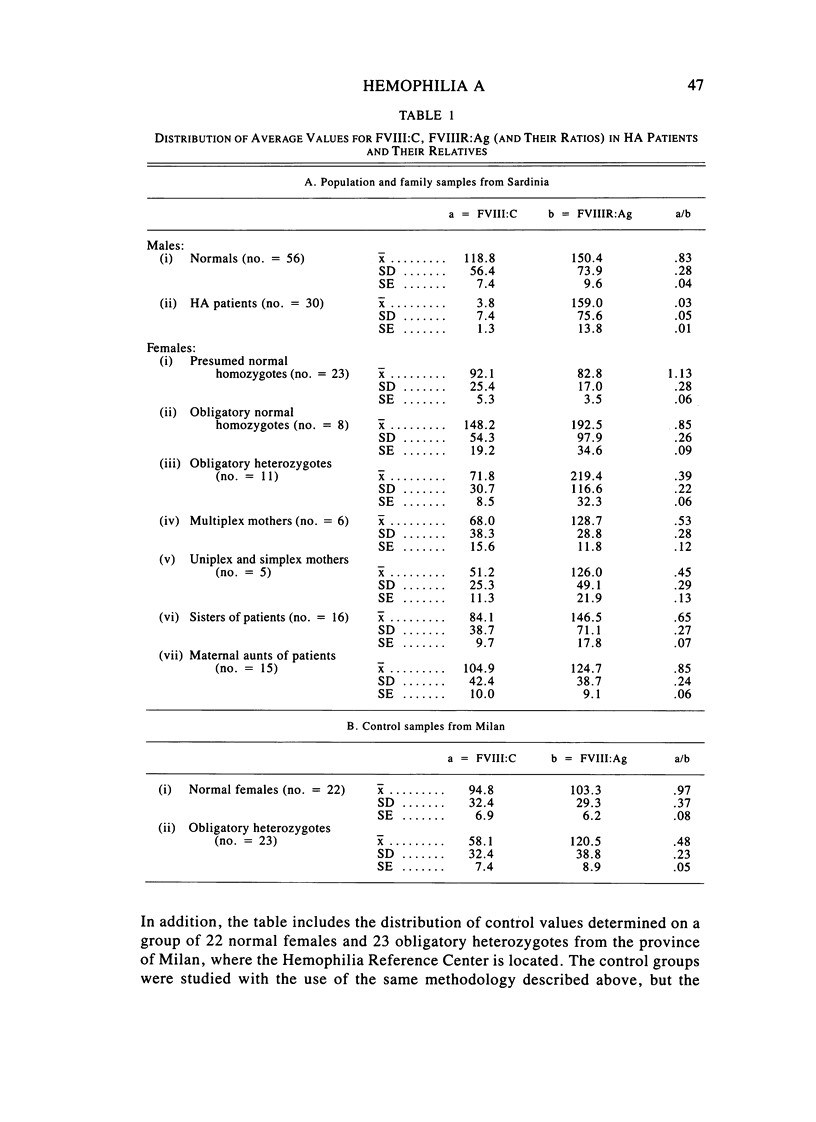
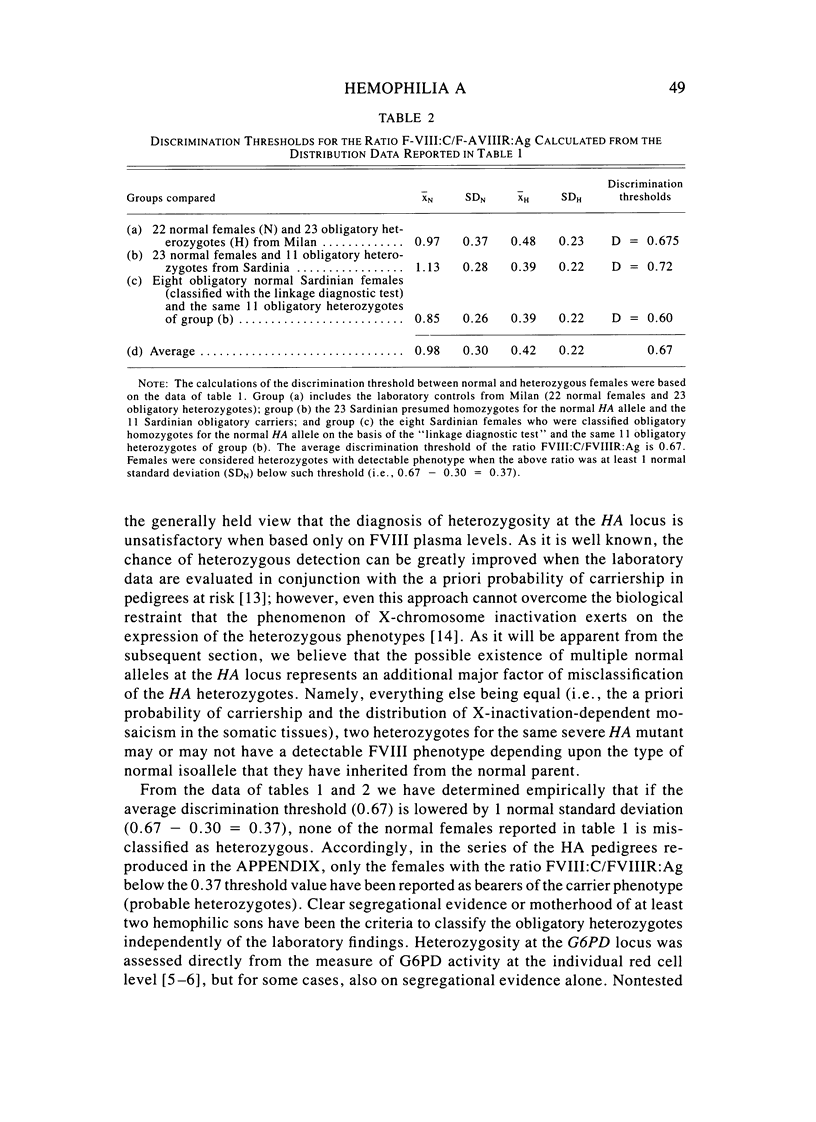
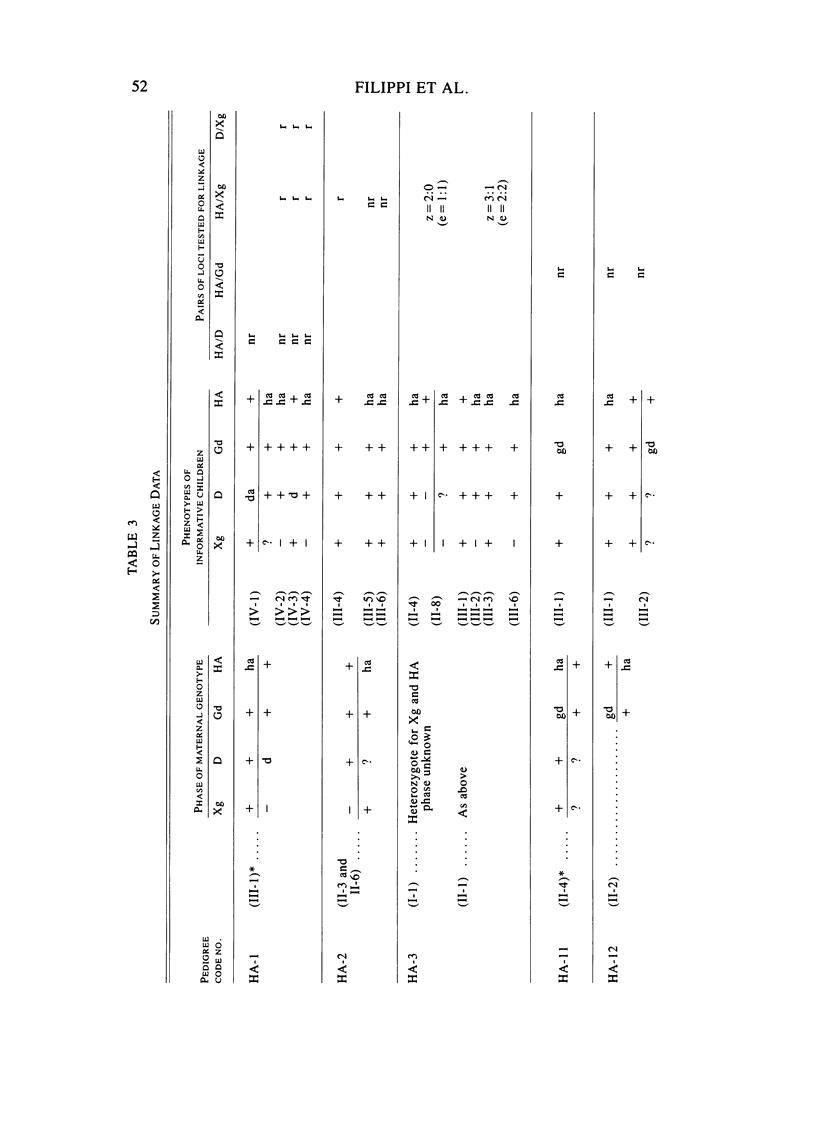
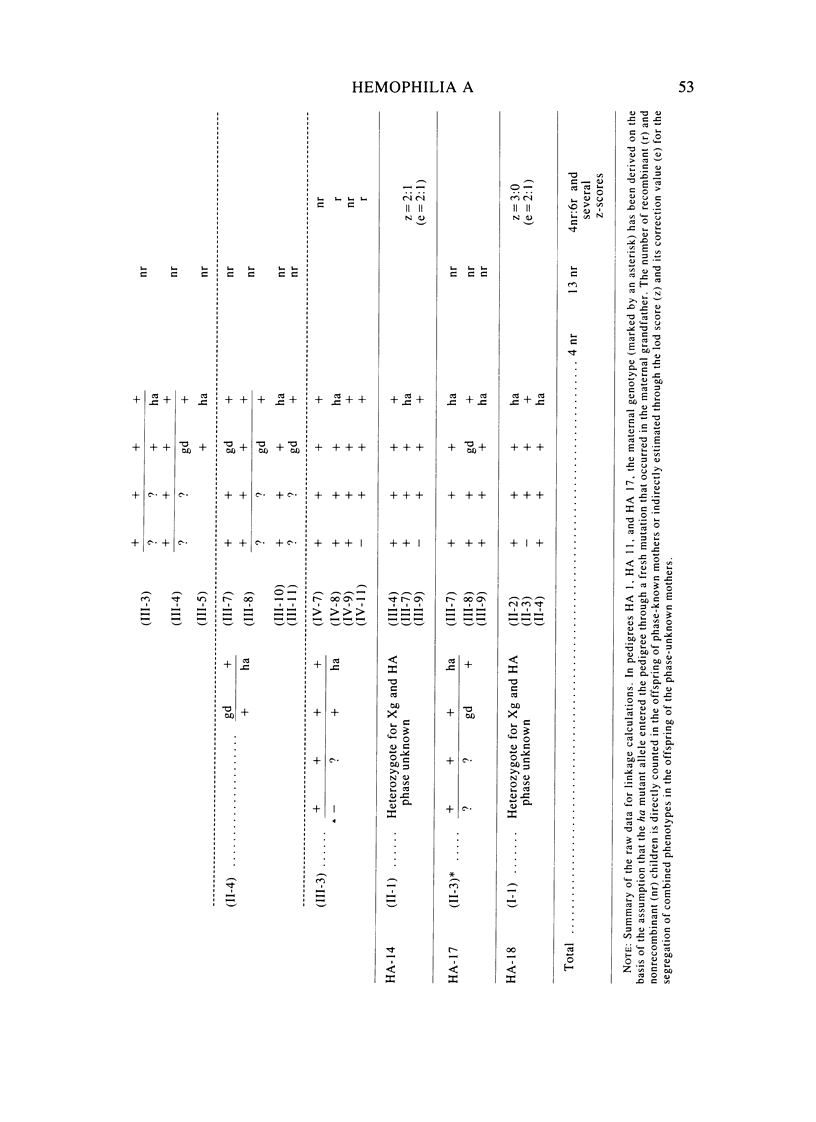
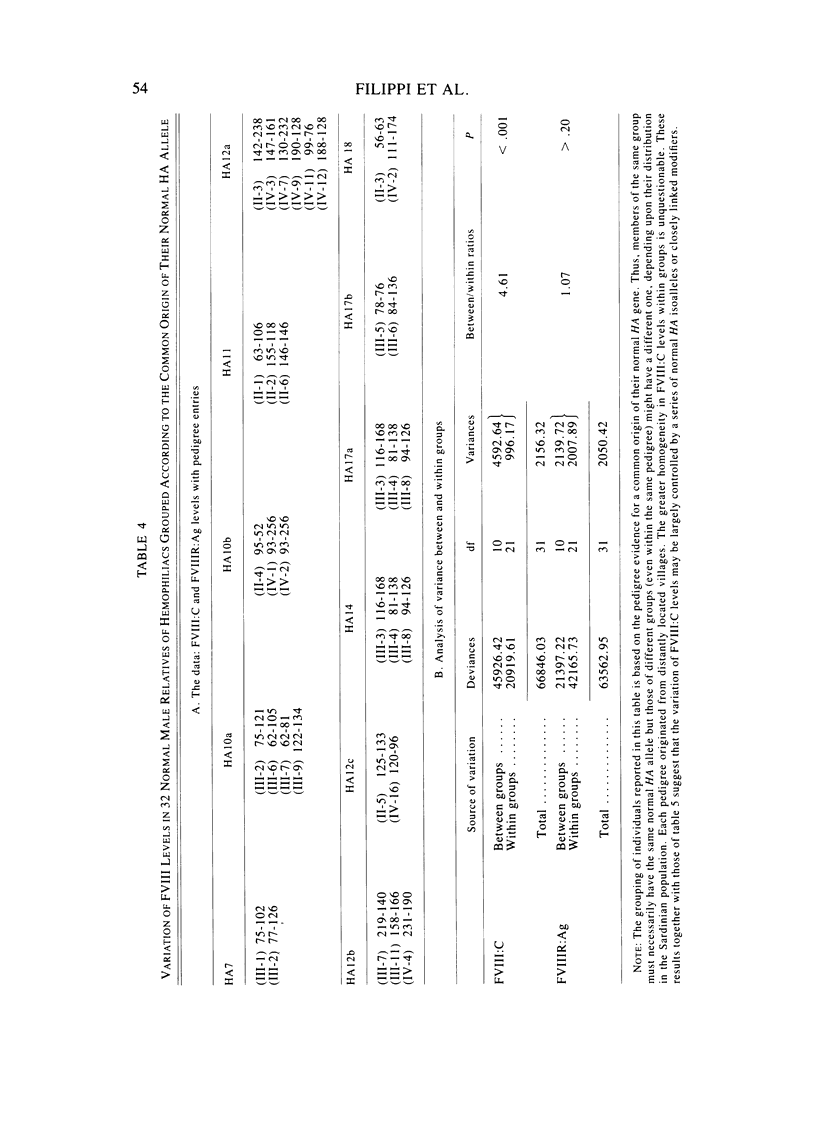
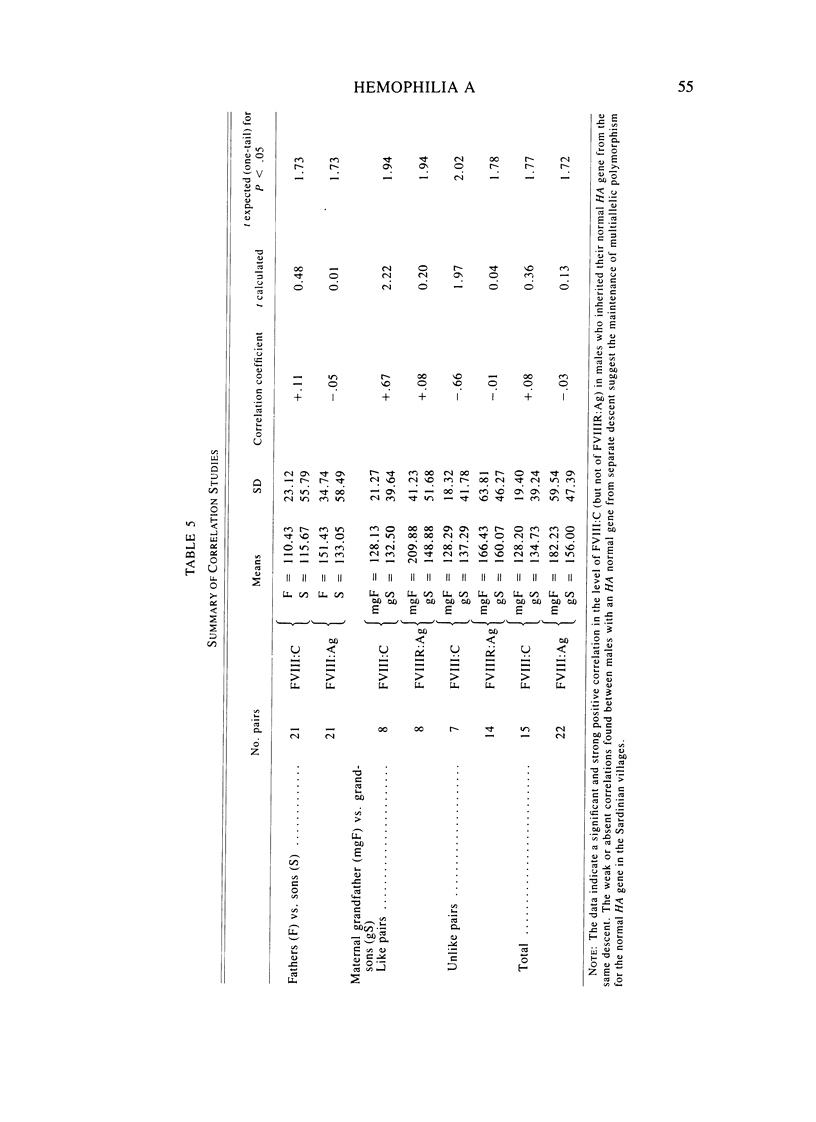
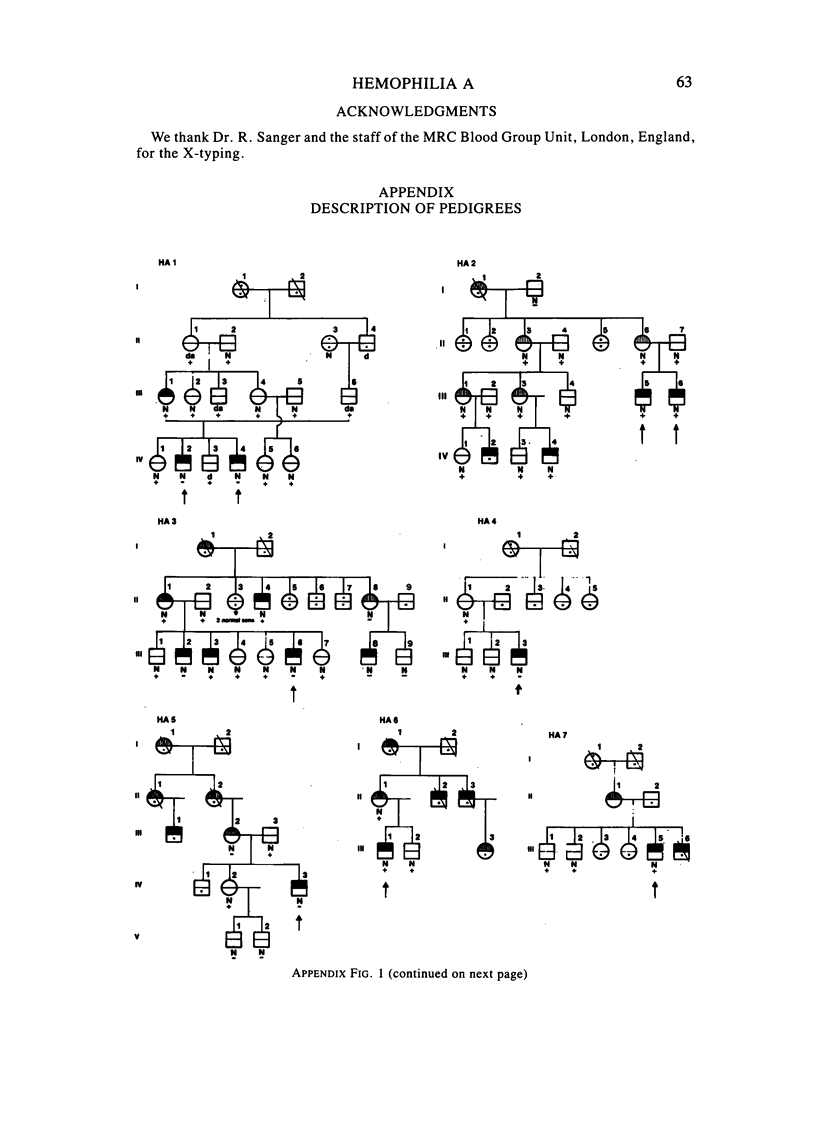
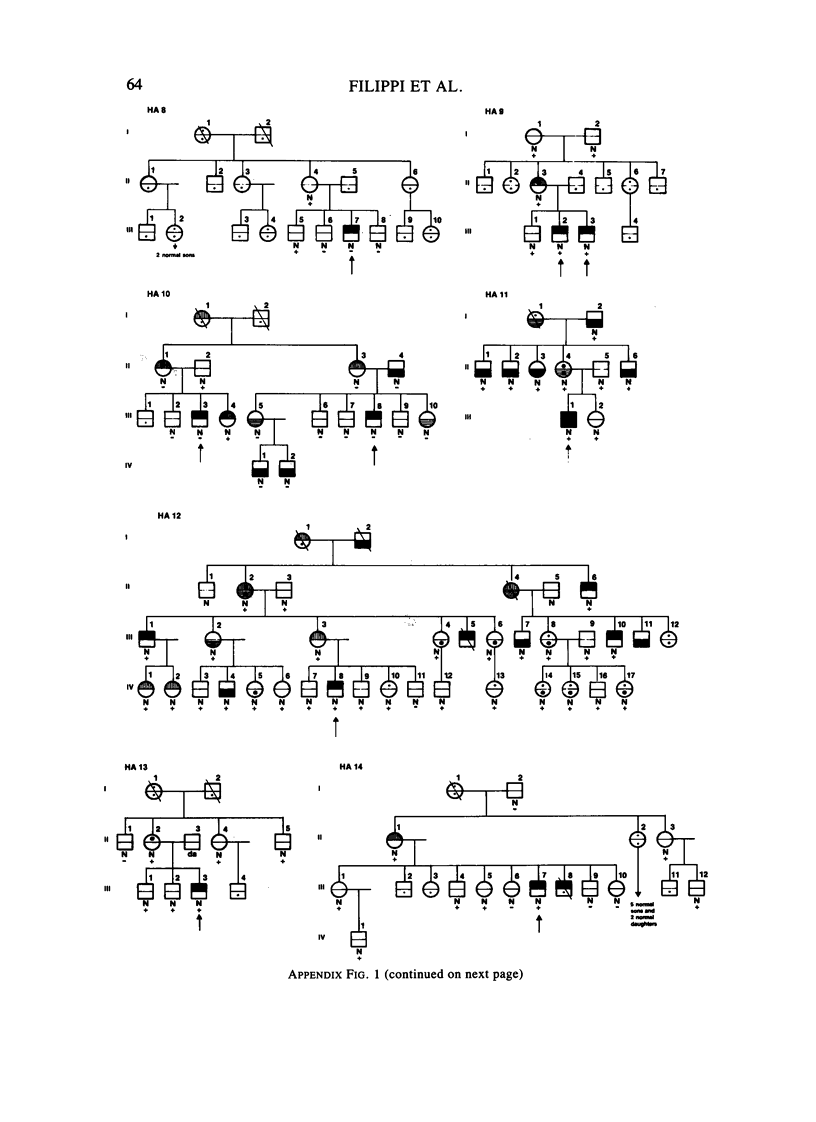
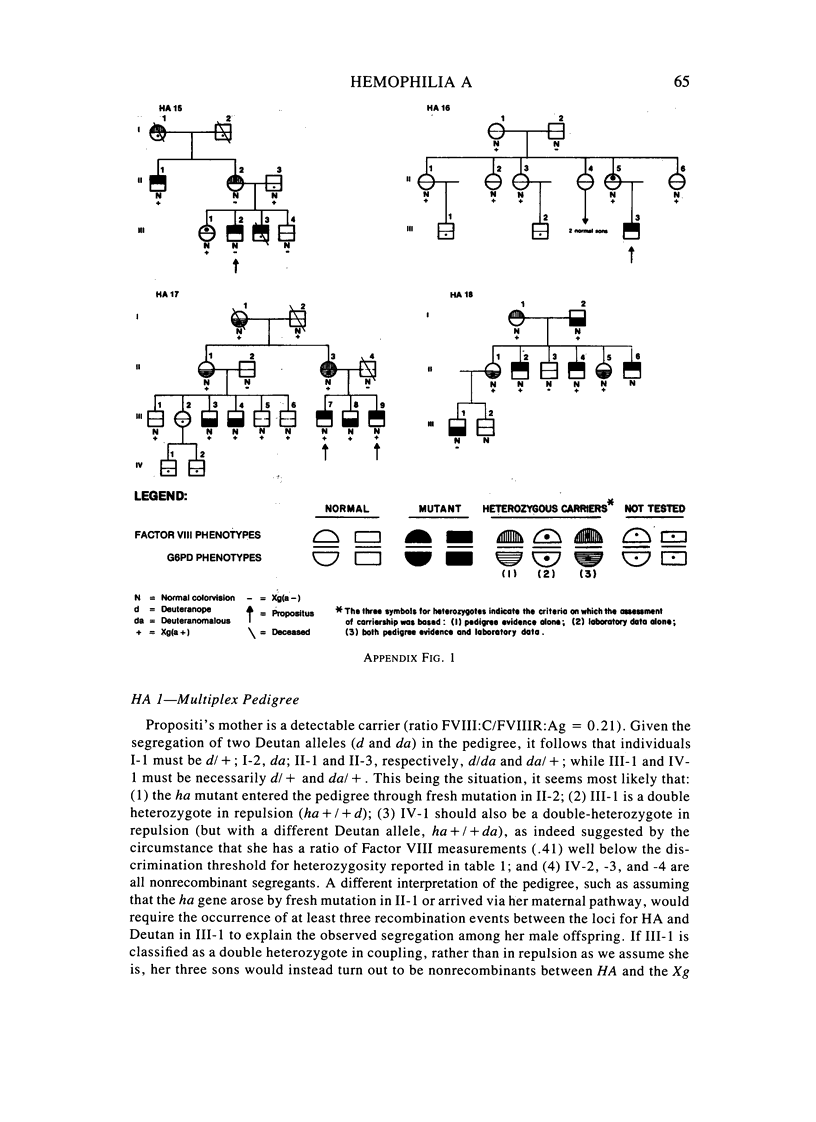
A large survey of hemophilia A carried out with almost complete ascertainment on the island of Sardinia suggests that the variation of plasma levels of Factor VIII coagulant activity in normal individuals is largely controlled by a series of normal isoalleles or by closely linked modifiers. This variation is expected to affect the laboratory detection of the hemophilia A (HA) heterozygotes in addition to the X-inactivation-dependent mosaicism and the type of deficient mutant present in a given pedigree. The Sardinian pedigrees yielded 13 new cases of nonrecombinants between the loci for HA and glucose-6-phosphate dehydrogenase (G6PD), as well as four nonrecombinants between HA and Deutan color blindness. These findings bring to a total of 58 the number of scorable sibs and nonrecombinants thus far known for the linkage HA-G6PD. From such a figure it has been possible to infer that the 90% upper limit of meiotic recombination between the two loci is below 4%, thus justifying the application of the "linkage diagnostic test" for the detection of HA heterozygotes and the prenatal diagnosis of the hemophilic fetuses in families that segregate at both loci. In three out of the five HA pedigrees of our series that segregate also for G6PD or Deutan color blindness, the observed segregation of the combined phenotypes can be best explained by assuming the occurrence of a fresh mutation in the maternal grandfathers. Such a finding points out the opportunity to reevaluate Haldane's hypothesis of a possible higher incidence of X-linked mutations in the human male. It is anticipated that each of the issues addressed by the present study will be amenable to experimental verification as soon as suitable molecular probes become available to screen for common multiallelic DNA polymorphisms in the subtelomeric region of the X-chromosome long arm.
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRINKHOUS K. M., LANGDELL R. D., PENICK G. D., GRAHAM J. B., WAGNER R. H. Newer approaches to the study of hemophilia and hemophilioid states. J Am Med Assoc. 1954 Feb 6;154(6):481–486. doi: 10.1001/jama.1954.02940400019005. [DOI] [PubMed] [Google Scholar]
- Barrai I., Cann H. M., Cavalli-Sforza L. L., De Nicola P. The effect of parental age on rates of mutation for hemophilia and evidence for differing mutation rates for hemophilia A and B. Am J Hum Genet. 1968 May;20(3):175–196. [PMC free article] [PubMed] [Google Scholar]
- Barrow E. M., Graham J. B. Blood coagulation factor VIII (antihemophilic factor): with comments on von Willebrand's disease and Christmas disease. Physiol Rev. 1974 Jan;54(1):23–74. doi: 10.1152/physrev.1974.54.1.23. [DOI] [PubMed] [Google Scholar]
- Bennett B., Ratnoff O. D. Detection of the carrier state for classic hemophilia. N Engl J Med. 1973 Feb 15;288(7):342–345. doi: 10.1056/NEJM197302152880704. [DOI] [PubMed] [Google Scholar]
- Boyer S. H., Graham J. B. Linkage Between the X Chromosome Loci for Glucose-6-Phosphate Dehydrogenase Electrophoretic Variation and Hemophilia A. Am J Hum Genet. 1965 Jul;17(4):320–324. [PMC free article] [PubMed] [Google Scholar]
- Brinkhous K. M., Davis P. D., Graham J. B., Dodds W. J. Expression and linkage of genes for X-linked hemophilias A and B in the dog. Blood. 1973 Apr;41(4):577–585. [PubMed] [Google Scholar]
- Cao A., Galanello R., Furbetta M., Muroni P. P., Garbato L., Rosatelli C., Scalas M. T., Addis M., Ruggeri R., Maccioni L. Thalassaemia types and their incidence in Sardinia. J Med Genet. 1978 Dec;15(6):443–447. doi: 10.1136/jmg.15.6.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES S. H., GAVIN J., GOLDSMITH K. L., GRAHAM J. B., HAMPER J., HARDISTY R. M., HARRIS J. B., HOLMAN C. A., INGRAM G. I., JONES T. G. THE LINKAGE RELATIONS OF HEMOPHILIA A AND HEMOPHILIA B (CHRISTMAS DISEASE) TO THE XG BLOOD GROUP SYSTEM. Am J Hum Genet. 1963 Dec;15:481–492. [PMC free article] [PubMed] [Google Scholar]
- Edgell C. J., Kirkman H. N., Clemons E., Buchanan P. D., Miller C. H. Prenatal diagnosis by linkage: hemophilia A and polymorphic glucose-6-phosphate deydrogenase. Am J Hum Genet. 1978 Jan;30(1):80–84. [PMC free article] [PubMed] [Google Scholar]
- GRAHAM J. B., MCLENDON W. W., BRINKHOUS K. M. Mild hemophilia; allelic form of the disease. Am J Med Sci. 1953 Jan;225(1):46–53. [PubMed] [Google Scholar]
- Gall J. C., Brewer G. J., Dern R. J. Studies of Glucose-6-Phosphate Dehydrogenase Activity of Individual Erythrocytes: The Methemoglobin-Elution Test for Identification of Females Heterozygous for G6PD Deficiency. Am J Hum Genet. 1965 Jul;17(4):359–368. [PMC free article] [PubMed] [Google Scholar]
- Gartler S. M., Gandini E., Angioni G., Argiolas N. Glucose-6 phosphate dehydrogenase mosaicism: utilization as a tracer in the study of the development of hair root cells. Ann Hum Genet. 1969 Oct;33(2):171–176. doi: 10.1111/j.1469-1809.1969.tb01642.x. [DOI] [PubMed] [Google Scholar]
- Graham J. B., Barrow E. S., Elston R. C. Lyonization in hemophilia: a cause of error in direct detection of heterozygous carriers. Ann N Y Acad Sci. 1975 Jan 20;240:141–146. doi: 10.1111/j.1749-6632.1975.tb53335.x. [DOI] [PubMed] [Google Scholar]
- KOSOWER N., CHRISTIANSEN R., MORTON N. E. Sporadic cases of hemophilia and the question of a possible sex difference in mutation rates. Am J Hum Genet. 1962 Jun;14:159–169. [PMC free article] [PubMed] [Google Scholar]
- Kerr C. B., Preston A. E., Barr A., Biggs R. Further studies on the inheritance of factor 8. Br J Haematol. 1966 Mar;12(2):212–233. doi: 10.1111/j.1365-2141.1966.tb05627.x. [DOI] [PubMed] [Google Scholar]
- LINDER D., GARTLER S. M. DISTRIBUTION OF GLUCOSE-6-PHOSPHATE DEHYDROGENASE ELECTROPHORETIC VARIANTS IN DIFFERENT TISSUES OF HETEROZYGOTES. Am J Hum Genet. 1965 May;17:212–220. [PMC free article] [PubMed] [Google Scholar]
- LYON M. F. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 1961 Apr 22;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- MORTON N. E. Further scoring types in sequential linkage tests, with a critical review of autosomal and partial sex linkage in man. Am J Hum Genet. 1957 Mar;9(1):55–75. [PMC free article] [PubMed] [Google Scholar]
- Methods for the detection of haemophilia carriers: a memorandum. Bull World Health Organ. 1977;55(6):675–702. [PMC free article] [PubMed] [Google Scholar]
- Miller O. J., Siniscalco M. Report of the committee on the genetic constitution of the x and y chromosomes. Oslo Conference (1981): Sixth International Workshop on Human Gene Mapping. Cytogenet Cell Genet. 1982;32(1-4):179–190. doi: 10.1159/000131697. [DOI] [PubMed] [Google Scholar]
- NANCE W. E. GENETIC TESTS WITH A SEX-LINKED MARKER: GLUCOSE-6-PHOSPHATE DEHYDROGENASE. Cold Spring Harb Symp Quant Biol. 1964;29:415–425. doi: 10.1101/sqb.1964.029.01.043. [DOI] [PubMed] [Google Scholar]
- Piomelli S., Siniscalco M. The haematological effects of glucose-6-phosphate dehydrogenase deficiency and thalassaemia trait: interaction between the two genes at the phenotype level. Br J Haematol. 1969 Jun;16(6):537–549. doi: 10.1111/j.1365-2141.1969.tb00435.x. [DOI] [PubMed] [Google Scholar]
- ROBERTSON J. H., TRUEMAN R. G. COMBINED HEMOPHILIA AND CHRISTMAS DISEASE. Blood. 1964 Sep;24:281–288. [PubMed] [Google Scholar]
- Rinaldi A., Filippi G., Siniscalco M. Variability of red cell phenotypes between and within individuals in an unbiased sample of 77 heterozygotes for G6PD deficiency in Sardinia. Am J Hum Genet. 1976 Sep;28(5):496–505. [PMC free article] [PubMed] [Google Scholar]
- Rinaldi A., Velivasakis M., Latte B., Filippi G., Siniscalco M. Triplo-X constitution of mother explains apparent occurrence of two recombinants in sibship segregating at two closely X-linked loci (G6PD and deutan). Am J Hum Genet. 1978 Jul;30(4):339–345. [PMC free article] [PubMed] [Google Scholar]
- Rizza C. R., Rhymes I. L., Austen D. E., Kernoff P. B., Aroni S. A. Detection of carriers of haemophilia: a 'blind' study. Br J Haematol. 1975 Aug;30(4):447–456. doi: 10.1111/j.1365-2141.1975.tb01859.x. [DOI] [PubMed] [Google Scholar]
- Roberts D. F. The genetic basis of variation in factor 8 levels among haemophiliacs. J Med Genet. 1971 Jun;8(2):136–139. doi: 10.1136/jmg.8.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri Z. M., Mannucci P. M., Jeffcoate S. L., Ingram G. I. Immunoradiometric assay of factor VIII related antigen, with observations in 32 patients with von Willebrand's disease. Br J Haematol. 1976 Jun;33(2):221–232. doi: 10.1111/j.1365-2141.1976.tb03533.x. [DOI] [PubMed] [Google Scholar]
- Siniscalco M., Bernini L., Filippi G., Latte B., Meera Khan P., Piomelli S., Rattazzi M. Population genetics of haemoglobin variants, thalassaemia and glucose-6-phosphate dehydrogenase deficiency, with particular reference to the malaria hypothesis. Bull World Health Organ. 1966;34(3):379–393. [PMC free article] [PubMed] [Google Scholar]
- Siniscalco M., Filippi G., Latte B., Piomelli S., Rattazzi M., Gavin J., Sanger R., Race R. R. Failure to detect linkage between Xg and other X-borne loci in Sardinians. Ann Hum Genet. 1966 Mar;29(3):231–252. doi: 10.1111/j.1469-1809.1966.tb00518.x. [DOI] [PubMed] [Google Scholar]
- WHITTAKER D. L., COPELAND D. L., GRAHAM J. B. Linkage of color blindness to hemophilias A and B. Am J Hum Genet. 1962 Jun;14:149–158. [PMC free article] [PubMed] [Google Scholar]
- Zimmerman T. S., Ratnoff O. D., Littell A. S. Detection of carriers of classic hemophilia using an immunologic assay for antihemophilic factor (factor 8(). J Clin Invest. 1971 Jan;50(1):255–258. doi: 10.1172/JCI106481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman T. S., Ratnoff O. D., Powell A. E. Immunologic differentiation of classic hemophilia (factor 8 deficiency) and von Willebrand's dissase, with observations on combined deficiencies of antihemophilic factor and proaccelerin (factor V) and on an acquired circulating anticoagulant against antihemophilic factor. J Clin Invest. 1971 Jan;50(1):244–254. doi: 10.1172/JCI106480. [DOI] [PMC free article] [PubMed] [Google Scholar]


