Abstract
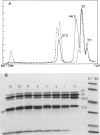
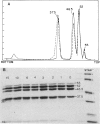
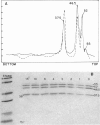
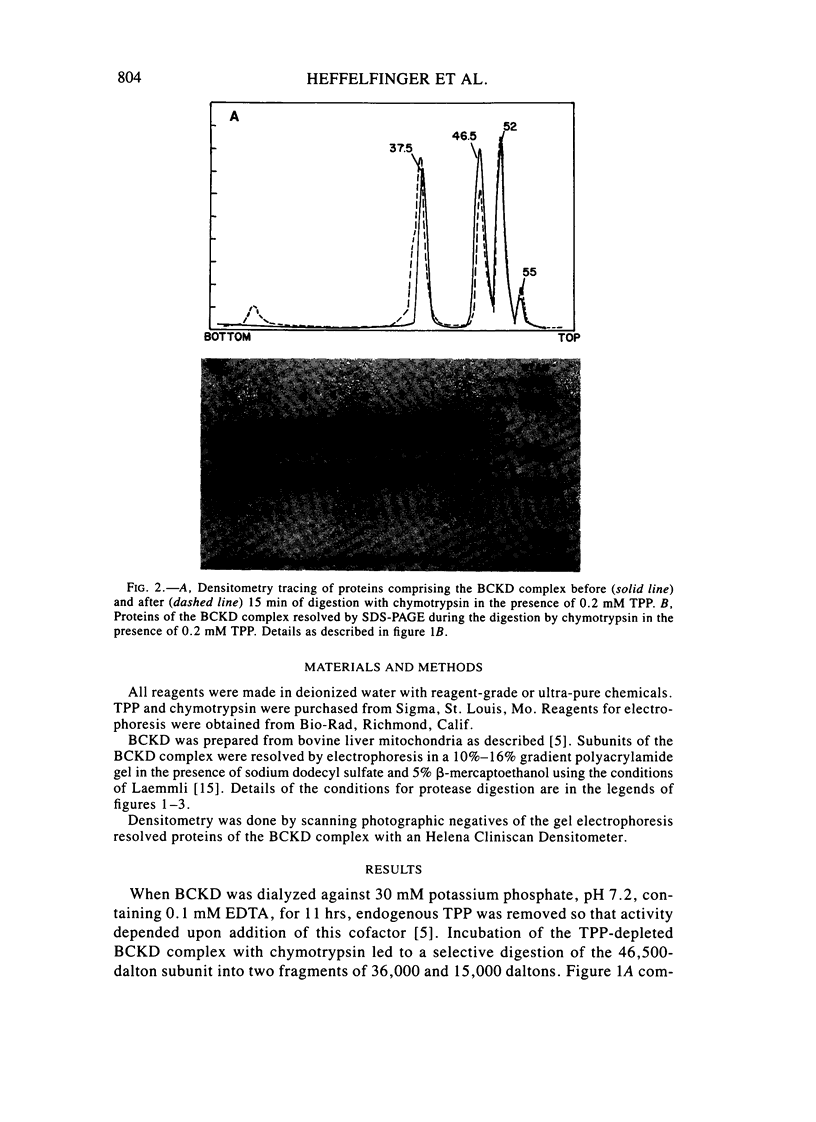
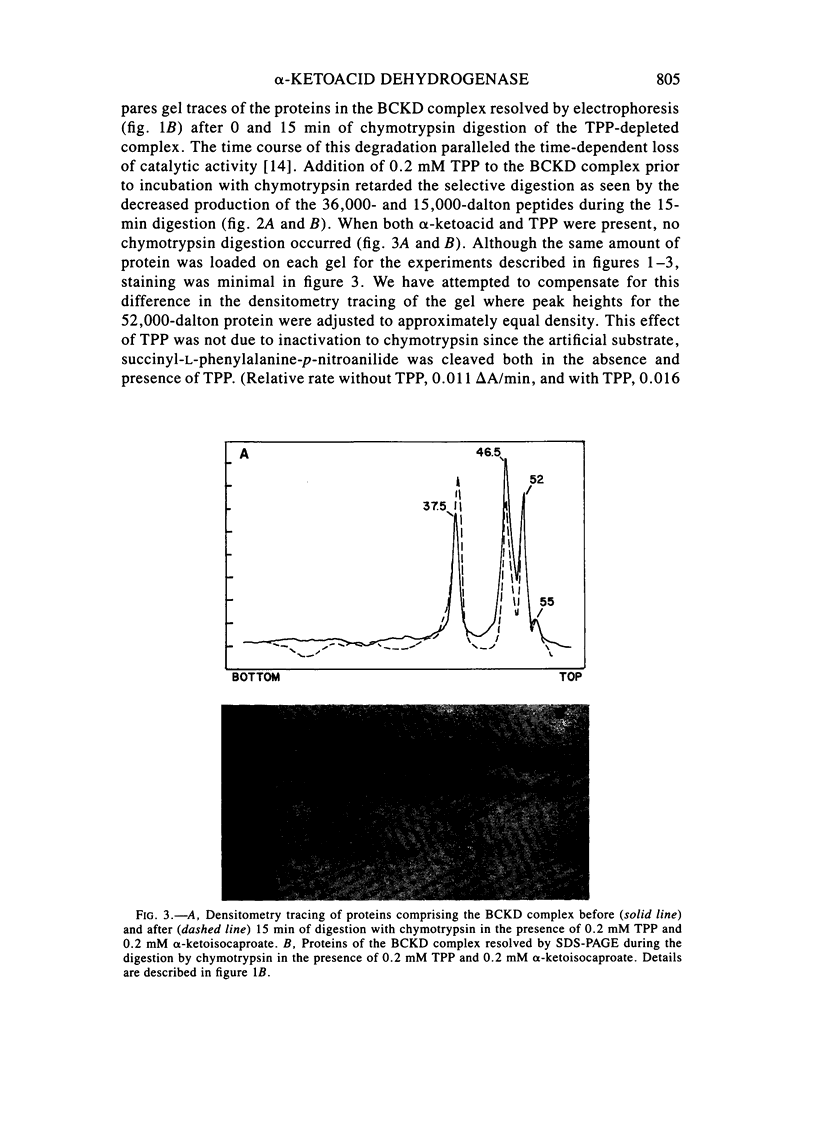
Branched-chain alpha-ketoacid dehydrogenase is a multienzyme complex composed of four subunits. The 46,500-dalton protein is a subunit of the decarboxylase component, which is selectively digested by chymotrypsin. Two peptides of apparent mol. wts. of 36,000 and 15,000 result with loss of enzyme activity. When the complex is saturated with thiamin pyrophosphate and ketoacid substrate, digestion by chymotrypsin does not occur. These data provide direct physical evidence for the stabilization of the complex by the presence of the vitamin B1-derived cofactor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buse M. G., Jursinic S., Reid S. S. Regulation of branched-chain amino acid oxidation in isolated muscles, nerves and aortas of rats. Biochem J. 1975 Jun;148(3):363–374. doi: 10.1042/bj1480363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner D. J., Davidson E. D., Elsas L. J., 2nd Thiamine increases the specific activity of human liver branched chain alpha-ketoacid dehydrogenase. Nature. 1975 Apr 10;254(5500):529–530. doi: 10.1038/254529a0. [DOI] [PubMed] [Google Scholar]
- Danner D. J., Lemmon S. K., Besharse J. C., Elsas L. J., 2nd Purification and characterization of branched chain alpha-ketoacid dehydrogenase from bovine liver mitochondria. J Biol Chem. 1979 Jun 25;254(12):5522–5526. [PubMed] [Google Scholar]
- Danner D. J., Lemmon S. K., Elsas L. J., 2nd Stabilization of mammalian liver branched-chain alpha-ketoacid dehydrogenase by thiamin pyrophosphate. Arch Biochem Biophys. 1980 Jun;202(1):23–28. doi: 10.1016/0003-9861(80)90401-4. [DOI] [PubMed] [Google Scholar]
- Danner D. J., Wheeler F. B., Lemmon S. K., Elsas L. J., 2nd In vivo and in vitro response of human branched chain alpha-ketoacid dehydrogenase to thiamine and thiamine pyrophosphate. Pediatr Res. 1978 Mar;12(3):235–238. doi: 10.1203/00006450-197803000-00016. [DOI] [PubMed] [Google Scholar]
- Duran M., Tielens A. G., Wadman S. K., Stigter J. C., Kleijer W. J. Effects of thiamine in a patient with a variant form of branched-chian ketoaciduria. Acta Paediatr Scand. 1978 May;67(3):367–372. doi: 10.1111/j.1651-2227.1978.tb16336.x. [DOI] [PubMed] [Google Scholar]
- Elsas L. J., 2nd, Danner D. J. The role of thiamin in maple syrup urine disease. Ann N Y Acad Sci. 1982;378:404–421. doi: 10.1111/j.1749-6632.1982.tb31214.x. [DOI] [PubMed] [Google Scholar]
- GREENGARD O., GORDON M. THE COFACTOR-MEDIATED REGULATION OF APOENZYME LEVELS IN ANIMAL TISSUES. I. THE PYRIDOXINE-INDUCED RISE OF RAT LIVER TYROSINE TRANSAMINASE LEVEL IN VIVO. J Biol Chem. 1963 Nov;238:3708–3710. [PubMed] [Google Scholar]
- Hayashi S. I., Granner D. K., Tomkins G. M. Tyrosine aminotransferase. Purificaton and characterization. J Biol Chem. 1967 Sep 25;242(18):3998–4006. [PubMed] [Google Scholar]
- Heffelfinger S. C., Sewell E. T., Danner D. J. Identification of specific subunits of highly purified bovine liver branched-chain ketoacid dehydrogenase. Biochemistry. 1983 Nov 22;22(24):5519–5522. doi: 10.1021/bi00293a011. [DOI] [PubMed] [Google Scholar]
- Hutson S. M., Cree T. C., Harper A. E. Regulation of leucine and alpha-ketoisocaproate metabolism in skeletal muscle. J Biol Chem. 1978 Nov 25;253(22):8126–8133. [PubMed] [Google Scholar]
- Kim Y. J., Rosenberg L. E. On the mechanism of pyridoxine responsive homocystinuria. II. Properties of normal and mutant cystathionine beta-synthase from cultured fibroblasts. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4821–4825. doi: 10.1073/pnas.71.12.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Longhi R. C., Fleisher L. D., Tallan H. H., Gaull G. E. Cystathionine beta-synthase deficiency: a qualitative abnormality of the deficient enzyme modified by vitamin B6 therapy. Pediatr Res. 1977 Feb;11(2):100–103. doi: 10.1203/00006450-197702000-00003. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Yeaman S. J., Reed L. J. Purification and characterization of branched chain alpha-keto acid dehydrogenase complex of bovine kidney. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4881–4885. doi: 10.1073/pnas.75.10.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueschel S. M., Bresnan M. J., Shih V. E., Levy H. L. Thiamine-responsive intermittent branched-chain ketoaciduria. J Pediatr. 1979 Apr;94(4):628–631. doi: 10.1016/s0022-3476(79)80036-0. [DOI] [PubMed] [Google Scholar]
- Scriver C. R., Mackenzie S., Clow C. L., Delvin E. Thiamine-responsive maple-syrup-urine disease. Lancet. 1971 Feb 13;1(7694):310–312. doi: 10.1016/s0140-6736(71)91041-5. [DOI] [PubMed] [Google Scholar]