Abstract
We have studied a kindred in which two parts of siblings, maternal first cousins, have a form of "minimal" androgen insensitivity that permits morphogenesis of unambiguous male external genitalia, but interferes with normal virilization at puberty. All four had gynecomastia that required reductive surgery. Apart from this common phenotype, they varied considerably in the temporal and regional aspects of their subvirilization and appreciably in their androgenic responsiveness to pharmacological doses of testosterone. The cultured genital skin fibroblasts from one set of siblings have an androgen-receptor activity with the following properties: (1) a normal maximum-binding capacity (Bmax) with 5 alpha-dihydrotestosterone (DHT), or the synthetic androgen, methyltrienolone (MT; R1881) as ligand; (2) a higher than normal apparent equilibrium dissociation constant (Kd) for DHT (0.77 nM) but not for MT; and (3) an elevated dissociation rate (k) of DHT-receptor (0.013 min-1, 37 degrees C), but not MT-receptor, complexes within intact cells. In addition, prolonged incubation with MT, but not DHT, augments the specific androgen-binding activity of the mutant cells as much as that of the controls. Normal cells yield lower values of apparent Kd for DHT (0.1-0.3 nM) after 2- than after 0.5-hr incubation (0.3-1.8 nM), and 1-hr values are intermediate. This occurs despite concurrent catabolic consumption of DHT from the medium and is considered to reflect transformation of initial, low-affinity DHT-receptor complexes to subsequent, higher-affinity states by a process that depends on time and initial ligand concentration. The mutant complexes described here can readily attain the highest state of affinity with MT, but have an impeded, variably expressed ability to do so with DHT. These findings suggest that a structural mutation at the X-linked locus that encodes the androgen-receptor protein is responsible for its androgen-selective dysfunction. Synthetic, nonhepatotoxic androgens, with corrective effects in vitro comparable to those of MT, may be therapeutically useful for these subjects.
Full text
PDF




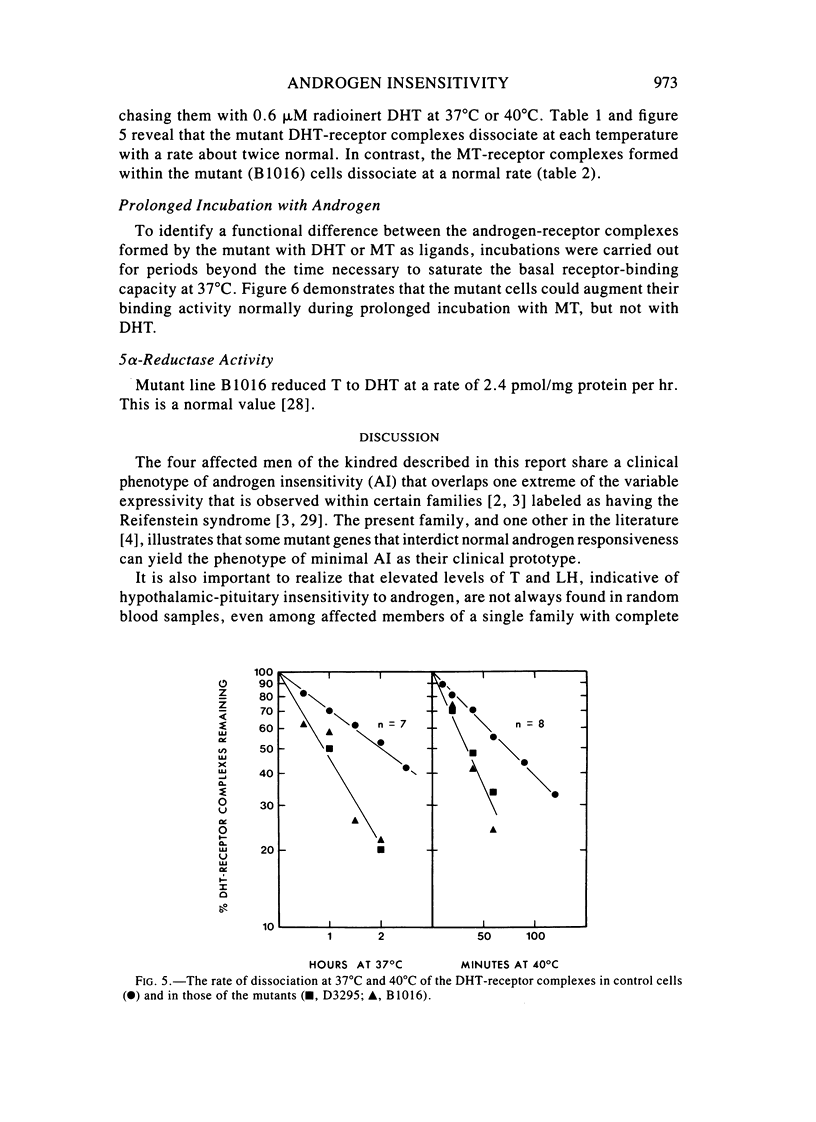
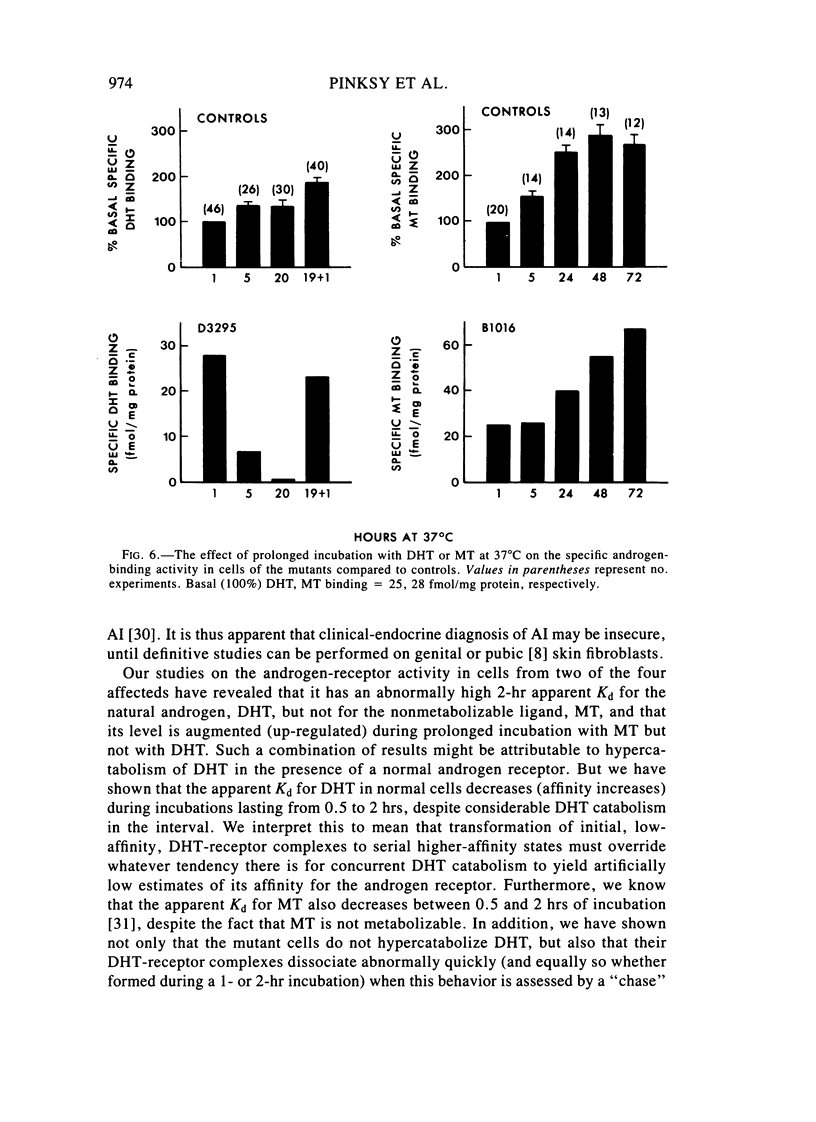




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiman J., Griffin J. E., Gazak J. M., Wilson J. D., MacDonald P. C. Androgen insensitivity as a cause of infertility in otherwise normal men. N Engl J Med. 1979 Feb 1;300(5):223–227. doi: 10.1056/NEJM197902013000503. [DOI] [PubMed] [Google Scholar]
- Aiman J., Griffin J. E. The frequency of androgen receptor deficiency in infertile men. J Clin Endocrinol Metab. 1982 Apr;54(4):725–732. doi: 10.1210/jcem-54-4-725. [DOI] [PubMed] [Google Scholar]
- Albert A., Rosemberg E., Ross G. T., Paulsen C. A., Ryan R. J. Report of the National Pituitary Agency collaborative study on the radioimmunoassay of FSH and LH. J Clin Endocrinol Metab. 1968 Aug;28(8):1214–1219. doi: 10.1210/jcem-28-8-1214. [DOI] [PubMed] [Google Scholar]
- Amrhein J. A., Klingensmith G. J., Walsh P. C., McKusick V. A., Migeon C. J. Partial androgen insensitivity: the Reifenstein syndrome revisited. N Engl J Med. 1977 Aug 18;297(7):350–356. doi: 10.1056/NEJM197708182970703. [DOI] [PubMed] [Google Scholar]
- Amrhein J. A., Meyer W. J., 3rd, Jones H. W., Jr, Migeon C. J. Androgen insensitivity in man: evidence for genetic heterogeneity. Proc Natl Acad Sci U S A. 1976 Mar;73(3):891–894. doi: 10.1073/pnas.73.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOWEN P., LEE C. S., MIGEON C. J., KAPLAN N. M., WHALLEY P. J., MCKUSICK V. A., REINFENSTEIN E. C., Jr HEREDITARY MALE PSEUDOHERMAPHRODITISM WITH HYPOGONADISM, HYPOSPADIAS, AND GYNECOMASTIA: REIFENSTEIN'S SYNDROME. Ann Intern Med. 1965 Feb;62:252–270. doi: 10.7326/0003-4819-62-2-252. [DOI] [PubMed] [Google Scholar]
- Brown T. R., Maes M., Rothwell S. W., Migeon C. J. Human complete androgen insensitivity with normal dihydrotestosterone receptor binding capacity in cultured genital skin fibroblasts: evidence for a qualitative abnormality of the receptor. J Clin Endocrinol Metab. 1982 Jul;55(1):61–69. doi: 10.1210/jcem-55-1-61. [DOI] [PubMed] [Google Scholar]
- Brown T. R., Rothwell S. W., Migeon C. J. Comparison of methyltrienolone and dihydrotestosterone binding and metabolism in human genital skin fibroblasts. J Steroid Biochem. 1981 Oct;14(10):1013–1022. doi: 10.1016/0022-4731(81)90209-0. [DOI] [PubMed] [Google Scholar]
- Eil C. Familial incomplete male pseudohermaphroditism associated with impaired nuclear androgen retention. Studies in cultured skin fibroblasts. J Clin Invest. 1983 Apr;71(4):850–858. doi: 10.1172/JCI110839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama S., Mayes D. M., Nugent C. A. A radioimmunoassay for plasma testosterone. Steroids. 1970 Oct;16(4):415–428. doi: 10.1016/s0039-128x(70)80124-6. [DOI] [PubMed] [Google Scholar]
- Griffin J. E. Testicular feminization associated with a thermolabile androgen receptor in culutred human fibroblasts. J Clin Invest. 1979 Dec;64(6):1624–1631. doi: 10.1172/JCI109624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. E., Wilson J. D. Studies on the pathogenesis of the incomplete forms of androgen resistance in man. J Clin Endocrinol Metab. 1977 Dec;45(6):1137–1143. doi: 10.1210/jcem-45-6-1137. [DOI] [PubMed] [Google Scholar]
- Griffin J. E., Wilson J. D. The syndromes of androgen resistance. N Engl J Med. 1980 Jan 24;302(4):198–209. doi: 10.1056/NEJM198001243020404. [DOI] [PubMed] [Google Scholar]
- Hodgins M. B. Binding of androgens in 5 alpha-reductase-deficient human genital skin fibroblasts: inhibition by progesterone and its metabolites. J Endocrinol. 1982 Sep;94(3):415–427. doi: 10.1677/joe.0.0940415. [DOI] [PubMed] [Google Scholar]
- Imperato-McGinley J., Peterson R. E., Gautier T., Cooper G., Danner R., Arthur A., Morris P. L., Sweeney W. J., Shackleton C. Hormonal evaluation of a large kindred with complete androgen insensitivity: evidence for secondary 5 alpha-reductase deficiency. J Clin Endocrinol Metab. 1982 May;54(5):931–941. doi: 10.1210/jcem-54-5-931. [DOI] [PubMed] [Google Scholar]
- Kaufman M., Pinsky L., Feder-Hollander R. Defective up-regulation of the androgen receptor in human androgen insensitivity. Nature. 1981 Oct 29;293(5835):735–737. doi: 10.1038/293735a0. [DOI] [PubMed] [Google Scholar]
- Kaufman M., Pinsky L., Hollander R., Bailey J. D. Regulation of the androgen receptor by androgen in normal and androgen-resistant genital skin fibroblasts. J Steroid Biochem. 1983 Apr;18(4):383–390. doi: 10.1016/0022-4731(83)90055-9. [DOI] [PubMed] [Google Scholar]
- Kaufman M., Straisfeld C., Pinsky L. Expression of androgen-responsive properties in human skin fibroblast strains of genital and nongenital origin. Somatic Cell Genet. 1977 Jan;3(1):17–25. doi: 10.1007/BF01550984. [DOI] [PubMed] [Google Scholar]
- Krüskemper H. L., Noell G. Liver toxicity of a new anabolic agent: methyltrienolone (17-alpha-methyl-4,9,11-estratriene-17 beta-ol-3-one). Steroids. 1966 Jul;8(1):13–24. doi: 10.1016/0039-128x(66)90114-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larrea F., Benavides G., Scaglia H., Kofman-Alfaro S., Ferrusca E., Medina M., Pérez-Palacios G. Gynecomastia as a familial incomplete male pseudohermaphroditism type 1: a limited androgen resistance syndrome. J Clin Endocrinol Metab. 1978 Jun;46(6):961–970. doi: 10.1210/jcem-46-6-961. [DOI] [PubMed] [Google Scholar]
- Lindner H. R., Perel E., Friedlander A., Zeitlin A. Specificity of antibodies to ovarian hormones in relation to the steroid hapten to the peptide carrier. Steroids. 1972 Mar;19(3):357–375. doi: 10.1016/0039-128x(72)90076-1. [DOI] [PubMed] [Google Scholar]
- Migeon B. R., Brown T. R., Axelman J., Migeon C. J. Studies of the locus for androgen receptor: localization on the human X chromosome and evidence for homology with the Tfm locus in the mouse. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6339–6343. doi: 10.1073/pnas.78.10.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T., Brandon D., Rodbard D., Loriaux D. L., Lipsett M. B. Glucocorticoid receptor in circulating mononuclear leukocytes. Endocrinology. 1979 Feb;104(2):500–505. doi: 10.1210/endo-104-2-500. [DOI] [PubMed] [Google Scholar]
- Pinsky L., Kaufman M., Gil-Esteban C., Sumbulian D. Regulation of the androgen receptor in human genital skin fibroblasts, with a review of sex steroid receptor regulation by homologous and heterologous steroids. Can J Biochem Cell Biol. 1983 Jul;61(7):770–778. doi: 10.1139/o83-097. [DOI] [PubMed] [Google Scholar]
- Pinsky L., Kaufman M., Summitt R. L. Congenital androgen insensitivity due to a qualitatively abnormal androgen receptor. Am J Med Genet. 1981;10(1):91–99. doi: 10.1002/ajmg.1320100111. [DOI] [PubMed] [Google Scholar]
- Pinsky L., Miller J., Shanfield B., Watters G., Wolfe L. S. GM1 gangliosidosis in skin fibroblast culture: enzymatic differences between types 1 and 2 and observations on a third variant. Am J Hum Genet. 1974 Sep;26(5):563–577. [PMC free article] [PubMed] [Google Scholar]
- Seeley D. H., Costas P. D. Transformation of a rabbit progesterone receptor from an 8S form to 5.5S and 4S forms. Mol Cell Endocrinol. 1983 May;30(2):161–178. doi: 10.1016/0303-7207(83)90045-x. [DOI] [PubMed] [Google Scholar]
- Thorneycroft I. H., Ribeiro W. O., Stone S. C., Tillson S. A. A radioimmunoassay of androstenedione. Steroids. 1973 Jan;21(1):111–122. doi: 10.1016/0039-128x(73)90024-x. [DOI] [PubMed] [Google Scholar]
- Walker A. C., Stack E. M., Horsfall W. A. Familial male pseudohermaphroditism. Med J Aust. 1970 Jan 24;1(4):156–160. doi: 10.5694/j.1326-5377.1970.tb77782.x. [DOI] [PubMed] [Google Scholar]
- Weichman B. M., Notides A. C. Estradiol-binding kinetics of the activated and nonactivated estrogen receptor. J Biol Chem. 1977 Dec 25;252(24):8856–8862. [PubMed] [Google Scholar]
- Weinstein A., Lindner H. R., Friedlander A., Bauminger S. Antigenic complexes of steroid hormones formed by coupling to protein through position 7: preparation from 4 -3-oxosteroids and characterization of antibodies to testosterone and androstenedione. Steroids. 1972 Dec;20(6):789–812. doi: 10.1016/0039-128x(72)90058-x. [DOI] [PubMed] [Google Scholar]
- Wilson J. D. Dihydrotestosterone formation in cultured human fibroblasts. Comparison of cells from normal subjects and patients with familial incomplete male pseudohermaphroditism, Type 2. J Biol Chem. 1975 May 10;250(9):3498–3504. [PubMed] [Google Scholar]
- Wilson J. D., Harrod M. J., Goldstein J. L., Hemsell D. L., MacDonald P. C. Familial incomplete male pseudohermaphroditism, type 1. Evidence for androgen resistance and variable clinical manifestations in a family with the Reifenstein syndrome. N Engl J Med. 1974 May 16;290(20):1097–1103. doi: 10.1056/NEJM197405162902001. [DOI] [PubMed] [Google Scholar]
- Wu C. H., Lundy L. E. Radioimmunoassay of plasma estrogens. Steroids. 1971 Jul;18(1):91–111. doi: 10.1016/s0039-128x(71)80174-5. [DOI] [PubMed] [Google Scholar]