Abstract
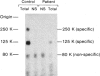
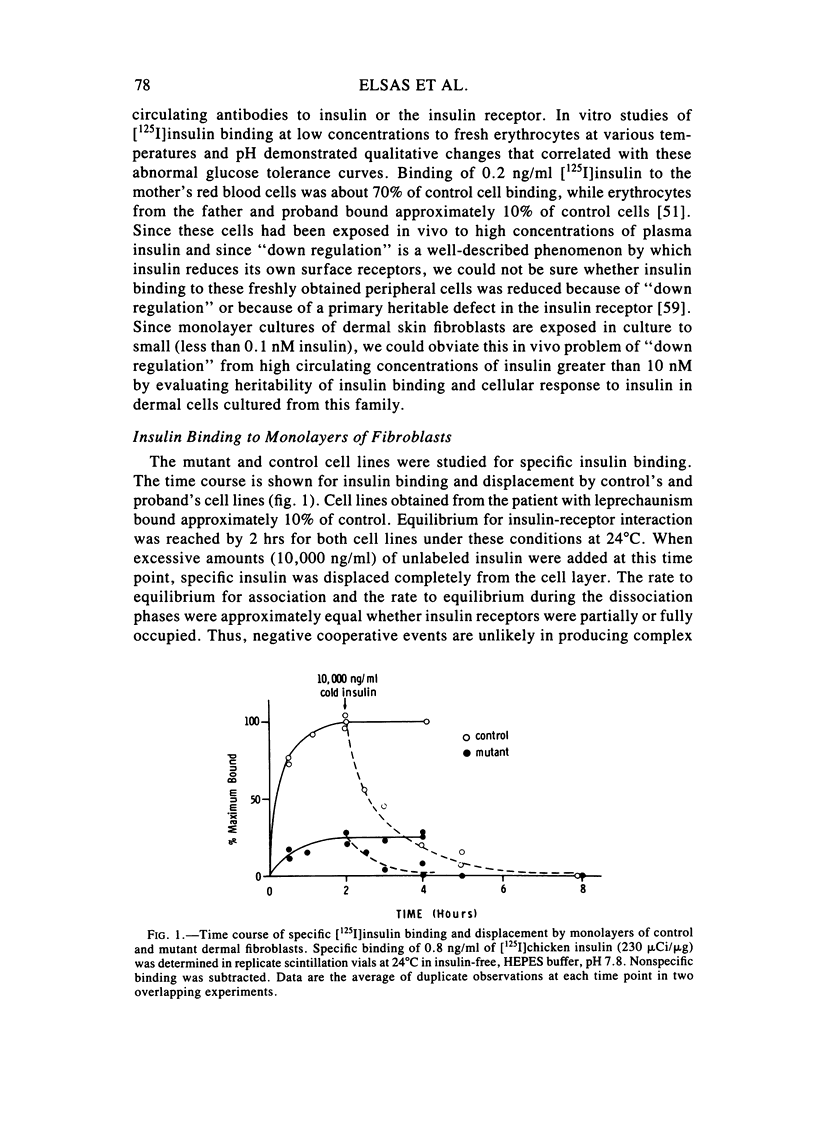
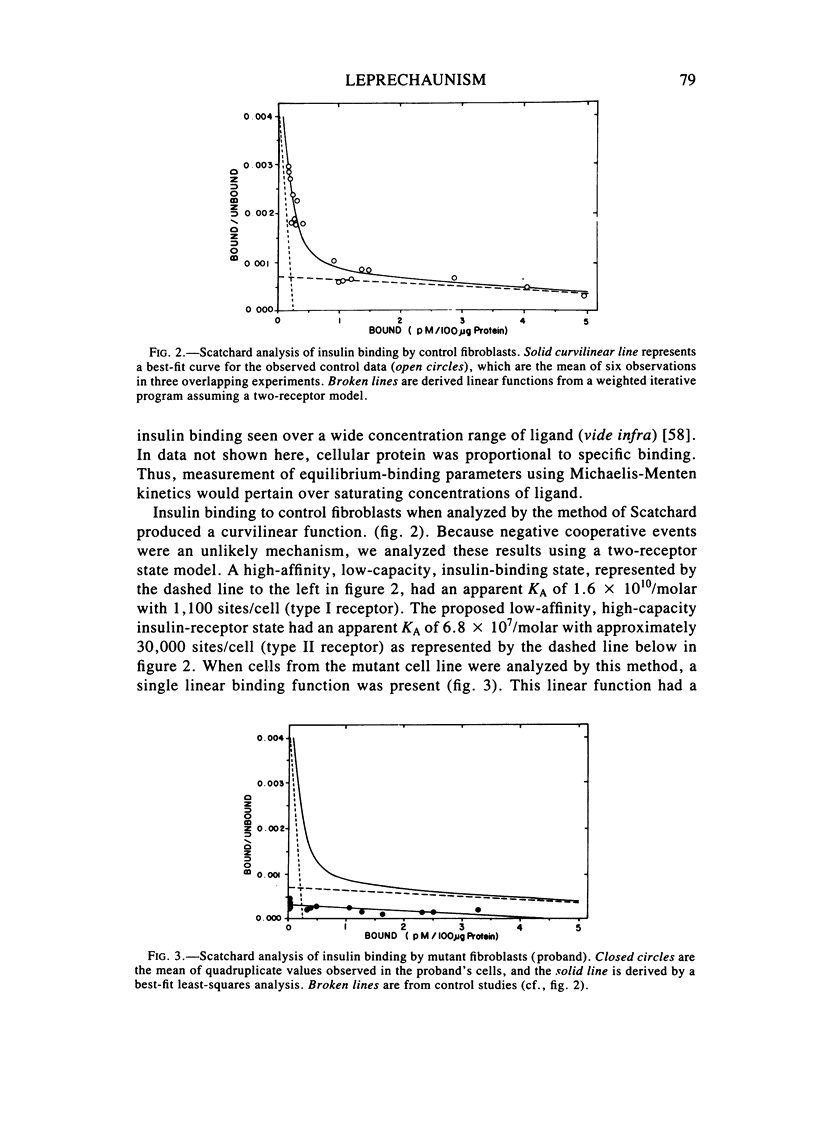
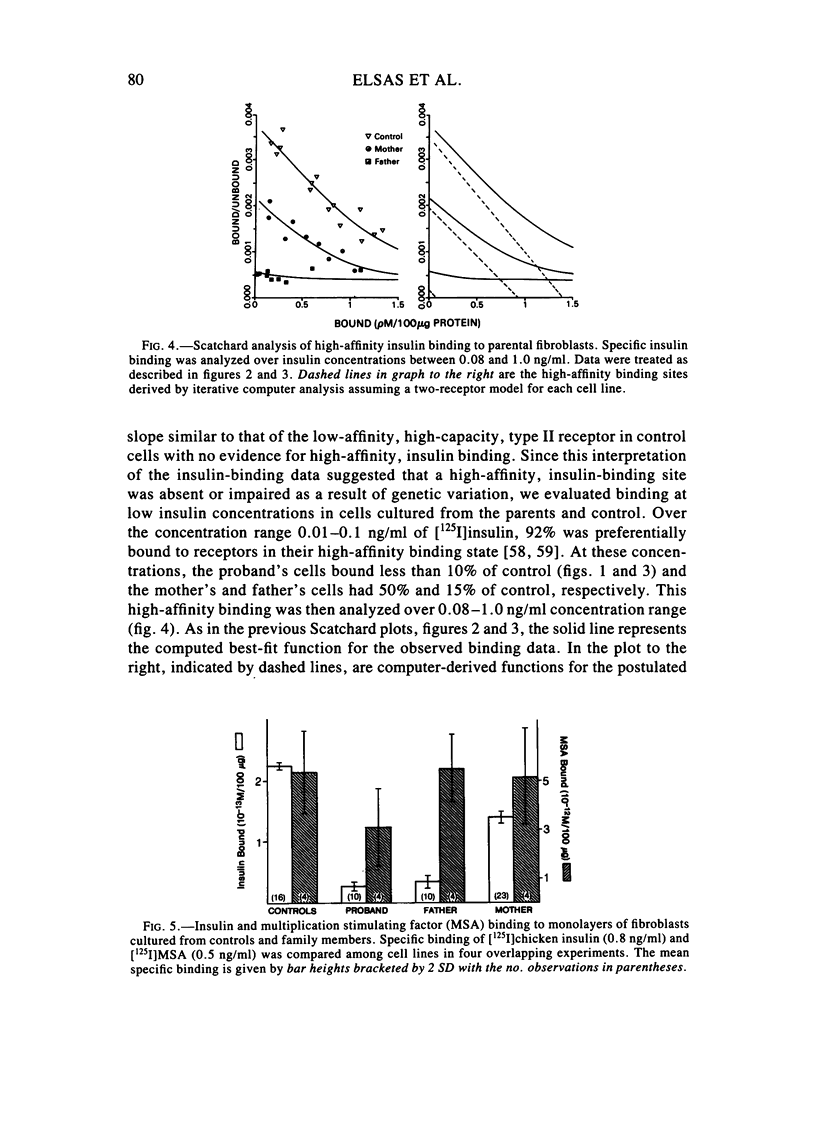
We examined in vivo oral glucose tolerance tests and in vitro insulin binding, cellular response, and insulin-receptor structure of fibroblasts cultured from the skin of a patient with leprechaun syndrome and her parents. In response to oral glucose, the proband exhibited marked hyperinsulinism (maximum plasma insulin = 4,120 microU/ml), the father had mild hyperinsulinism (maximum plasma insulin = 240 microU/ml), and the mother was normal. [125I]insulin binding to monolayers of intact fibroblasts demonstrated complex kinetics that were interpreted using a two-receptor model. Normal high-affinity binding had an apparent KA of 1.6 X 10(10)/molar with 1,100 sites/cell. The proposed low-affinity state receptor had an apparent KA of 6.8 X 10(7)/molar with approximately 30,000 sites/cell. Insulin binding to the proband's cells had no high-affinity binding but had normal low-affinity binding. Cells from the mother had 60%, and cells from the father, 2%, of control insulin binding to the high-affinity receptor, but normal, low-affinity site binding. Two different, insulin-stimulable responses were evaluated under experimental conditions identical with those used for insulin binding. Insulin stimulation of 2-methylaminoisobutyric acid uptake occurred with half-maximal responses between 25 and 50 ng/ml insulin. This response was similar in cells from controls and the patient. By contrast, the uptake and phosphorylation of 2-deoxy-D-glucose was stimulated at half-maximal insulin concentrations between 1 and 10 ng/ml in control cells but was not significantly increased in the proband's cells until 1,000 ng/ml concentrations of insulin were attained. In affinity crosslinking experiments, [125I]insulin was covalently bound to insulin receptors of fibroblast membranes using disuccinimidylsuberate. [125I]insulin specifically bound to 125,000 dalton monomeric subunits and 250,000 dalton dimers. In control cells, the ratio of monomer to dimer was approximately one, but significantly fewer dimers were crosslinked in insulin receptors from the patient's cells. We conclude that in this family two different recessive mutations impair high-affinity insulin-receptor binding and that the proband with leprechaunism is a compound heterozygote for these mutations. The two mutations produced structural changes in the receptor that altered subunit interactions and loss of high-affinity binding and cellular responsivity.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Gordon L. P., Dutton R. V., Rosenberg H. S., Rudolph A. J. Leprechaunism (Donohue's syndrome) in a low birth weight infant. South Med J. 1977 Aug;70(8):998–1001. doi: 10.1097/00007611-197708000-00031. [DOI] [PubMed] [Google Scholar]
- Bar R. S., Harrison L. C., Muggeo M., Gorden P., Kahn C. R., Roth J. Regulation of insulin receptors in normal and abnormal physiology in humans. Adv Intern Med. 1979;24:23–52. [PubMed] [Google Scholar]
- Bar R. S., Levis W. R., Rechler M. M., Harrison L. C., Siebert C., Podskalny J., Roth J., Muggeo M. Extreme insulin resistance in ataxia telangiectasia: defect in affinity of insulin receptors. N Engl J Med. 1978 May 25;298(21):1164–1171. doi: 10.1056/NEJM197805252982103. [DOI] [PubMed] [Google Scholar]
- Bier D. M., Schedewie H., Larner J., Olefsky J., Rubenstein A., Fiser R. H., Craig J. W., Elders M. J. Glucose kinetics in leprechaunism: accelerated fasting due to insulin resistance. J Clin Endocrinol Metab. 1980 Nov;51(5):988–994. doi: 10.1210/jcem-51-5-988. [DOI] [PubMed] [Google Scholar]
- Bode H. H., Crawford J. D. Nephrogenic diabetes insipidus in North America. The Hopewell hypothesis. N Engl J Med. 1969 Apr 3;280(14):750–754. doi: 10.1056/NEJM196904032801404. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Familial hypercholesterolemia: A genetic defect in the low-density lipoprotein receptor. N Engl J Med. 1976 Jun 17;294(25):1386–1390. doi: 10.1056/NEJM197606172942509. [DOI] [PubMed] [Google Scholar]
- Czech M. P. Cellular dynamics of insulin action. Fed Proc. 1982 Sep;41(11):2717–2718. [PubMed] [Google Scholar]
- Czech M. P., Massague J. Subunit structure and dynamics of the insulin receptor. Fed Proc. 1982 Sep;41(11):2719–2723. [PubMed] [Google Scholar]
- Czech M. P., Oppenheimer C. L., Massagué J. Interrelationships among receptor structures for insulin and peptide growth factors. Fed Proc. 1983 Jun;42(9):2598–2601. [PubMed] [Google Scholar]
- D'Ercole A. J., Underwood L. E., Groelke J., Plet A. Leprechaunism: studies of the relationship among hyperinsulinism, insulin resistance, and growth retardation. J Clin Endocrinol Metab. 1979 Mar;48(3):495–502. doi: 10.1210/jcem-48-3-495. [DOI] [PubMed] [Google Scholar]
- DONOHUE W. L., UCHIDA I. Leprechaunism: a euphemism for a rare familial disorder. J Pediatr. 1954 Nov;45(5):505–519. doi: 10.1016/s0022-3476(54)80113-2. [DOI] [PubMed] [Google Scholar]
- DeMeyts P., Bainco A. R., Roth J. Site-site interactions among insulin receptors. Characterization of the negative cooperativity. J Biol Chem. 1976 Apr 10;251(7):1877–1888. [PubMed] [Google Scholar]
- Dekaban A. Metabolic and chromosomal studies in leprechaunism. Arch Dis Child. 1965 Dec;40(214):632–636. doi: 10.1136/adc.40.214.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der Kaloustian V. M., Kronfol N. M., Takla R., Habash A., Khazin A., Najjar S. S. Leprechaunism. A report of two new cases. Am J Dis Child. 1971 Nov;122(5):442–445. [PubMed] [Google Scholar]
- Dunnigan M. G., Cochrane M. A., Kelly A., Scott J. W. Familial lipoatrophic diabetes with dominant transmission. A new syndrome. Q J Med. 1974 Jan;43(169):33–48. [PubMed] [Google Scholar]
- EVANS P. R. Leprechaunism. Arch Dis Child. 1955 Dec;30(154):479–483. doi: 10.1136/adc.30.154.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. A., Sethi P. K., Scoma A. J., Bannerman R. M., Frohman L. A. A new familial syndrome characterized by pigmentary retinopathy, hypogonadism, mental retardation, nerve deafness and glucose intolerance. Am J Med. 1976 Jan;60(1):23–32. doi: 10.1016/0002-9343(76)90529-5. [DOI] [PubMed] [Google Scholar]
- Elsas L. J., 2nd, MacDonell R. C., Jr, Rosenberg L. E. Influence of age on insulin stimulation of amino acid uptake in rat diaphragm. J Biol Chem. 1971 Nov;246(21):6452–6459. [PubMed] [Google Scholar]
- Elsas L. J., Wheeler F. B., Danner D. J., DeHaan R. L. Amino acid transport by aggregates of cultured chicken heart cells. Effect of insulin. J Biol Chem. 1975 Dec 25;250(24):9381–9390. [PubMed] [Google Scholar]
- Ferguson-Smith M. A., Hamilton W., Ferguson I. C., Ellis P. M. An abnormal metacentric chromosome in an infant with leprechaunism. Ann Genet. 1968 Dec;11(4):195–200. [PubMed] [Google Scholar]
- Fitch N. Albright's hereditary osteodystrophy: a review. Am J Med Genet. 1982 Jan;11(1):11–29. doi: 10.1002/ajmg.1320110104. [DOI] [PubMed] [Google Scholar]
- Franks R. C., Nance W. E. Hereditary adrenocortical unresponsiveness to ACTH. Pediatrics. 1970 Jan;45(1):43–48. [PubMed] [Google Scholar]
- Gillerot Y., Buez A., Hustin J. Leprechaunism (Donohue syndrome): a case with growth hormone deficiency. Acta Paediatr Belg. 1979 Oct-Dec;32(4):279–282. [PubMed] [Google Scholar]
- Goden P., Griggs R. C., Nissley S. P., Roth J., Engel W. K. Studies of plasma insulin in myotonic dystrophy. J Clin Endocrinol Metab. 1969 May;29(5):684–690. doi: 10.1210/jcem-29-5-684. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Fialkow P. J. The Alström syndrome. Report of three cases with further delineation of the clinical, pathophysiological, and genetic aspects of the disorder. Medicine (Baltimore) 1973 Jan;52(1):53–71. doi: 10.1097/00005792-197301000-00003. [DOI] [PubMed] [Google Scholar]
- Gross-Kieselstein E., Ben-Galim E., Amir N., Abrahamov A. Leprechaunism (Donohue syndrome). Am J Dis Child. 1973 Oct;126(4):500–503. doi: 10.1001/archpedi.1973.02110190410011. [DOI] [PubMed] [Google Scholar]
- Hartdegen R. G., Dogliotti M., Rabinowitz L., Bartlett R. G. Leprechaunism. Case report in a black African child. Br J Dermatol. 1975 Nov;93(5):587–591. doi: 10.1111/j.1365-2133.1975.tb02254.x. [DOI] [PubMed] [Google Scholar]
- Hill D. E. Effect of insulin on fetal growth. Semin Perinatol. 1978 Oct;2(4):319–328. [PubMed] [Google Scholar]
- Iwaski H., Abe M., Nawate G., Kato H. A case of chromosome abnormality (46, XX, Gq+) with congenital heart disease and leprechaunism. Jinrui Idengaku Zasshi. 1974 Jun;19(1):82–83. [PubMed] [Google Scholar]
- KALLO A., LAKATOS I., SZIJARTO L. LEPRECHAUNISM (DONOHUE'S SYNDROME). J Pediatr. 1965 Feb;66:372–379. doi: 10.1016/s0022-3476(65)80195-0. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Flier J. S., Bar R. S., Archer J. A., Gorden P., Martin M. M., Roth J. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med. 1976 Apr 1;294(14):739–745. doi: 10.1056/NEJM197604012941401. [DOI] [PubMed] [Google Scholar]
- Kahn C. R. Membrane receptors for hormones and neurotransmitters. J Cell Biol. 1976 Aug;70(2 Pt 1):261–286. doi: 10.1083/jcb.70.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplowitz P. B., D'Ercole A. J. Fibroblasts from a patient with leprechaunism are resistant to insulin, epidermal growth factor, and somatomedin C. J Clin Endocrinol Metab. 1982 Oct;55(4):741–748. doi: 10.1210/jcem-55-4-741. [DOI] [PubMed] [Google Scholar]
- King G. L., Rechler M. M., Kahn C. R. Interactions between the receptors for insulin and the insulin-like growth factors on adipocytes. J Biol Chem. 1982 Sep 10;257(17):10001–10006. [PubMed] [Google Scholar]
- Kobayashi M., Olefsky J. M., Elders J., Mako M. E., Given B. D., Schedwie H. K., Fiser R. H., Hintz R. L., Horner J. A., Rubenstein A. H. Insulin resistance due to a defect distal to the insulin receptor: demonstration in a patient with leprechaunism. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3469–3473. doi: 10.1073/pnas.75.7.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koontz J. W., Iwahashi M. Insulin as a potent, specific growth factor in a rat hepatoma cell line. Science. 1981 Feb 27;211(4485):947–949. doi: 10.1126/science.7008195. [DOI] [PubMed] [Google Scholar]
- Laron Z. The syndrome of familial dwarfism and high plasma immunoreactive human growth hormone. Birth Defects Orig Artic Ser. 1974;10(4):231–238. [PubMed] [Google Scholar]
- Massagué J., Blinderman L. A., Czech M. P. The high affinity insulin receptor mediates growth stimulation in rat hepatoma cells. J Biol Chem. 1982 Dec 10;257(23):13958–13963. [PubMed] [Google Scholar]
- Massagué J., Czech M. P. The subunit structures of two distinct receptors for insulin-like growth factors I and II and their relationship to the insulin receptor. J Biol Chem. 1982 May 10;257(9):5038–5045. [PubMed] [Google Scholar]
- Menon P. S., Sachdev H. P., Verma I. C. Diagnostic criteria in leprechaunism (Donohue syndrome). Indian J Pediatr. 1980 Mar-Apr;47(385):161–164. doi: 10.1007/BF02822886. [DOI] [PubMed] [Google Scholar]
- Migeon C. J., Kenny E. M., Kowarski A., Snipes C. A., Spaulding J. S., Finkelstein J. W., Blizzard R. M. The syndrome of congenital adrenocortical unresponsiveness to ACTH. Report of six cases. Pediatr Res. 1968 Nov;2(6):501–513. doi: 10.1203/00006450-196811000-00008. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. LIlly lecture 1980. Insulin resistance and insulin action. An in vitro and in vivo perspective. Diabetes. 1981 Feb;30(2):148–162. doi: 10.2337/diab.30.2.148. [DOI] [PubMed] [Google Scholar]
- Ordway N. K., Stout L. C. Intrauterine growth retardation, jaundice, and hypoglycemia in a neonate. J Pediatr. 1973 Nov;83(5):867–874. doi: 10.1016/s0022-3476(73)80391-9. [DOI] [PubMed] [Google Scholar]
- PATTERSON J. H., WATKINS W. L. Leprechaunism in a male infant. J Pediatr. 1962 May;60:730–739. doi: 10.1016/s0022-3476(62)80100-0. [DOI] [PubMed] [Google Scholar]
- Poradovska W., Jaworska M., Hanc I. Donohue's syndrome (leprechaunism) in a female infant. Acta Chir Plast. 1972;14(1):4–12. [PubMed] [Google Scholar]
- Ramaat L. G., Shafi K. M., Dajani F. F. Donohue's syndrome (report of a case). Indian Pediatr. 1977 Apr;14(4):325–327. [PubMed] [Google Scholar]
- Rechler M. M. Leprechaunism and related syndromes with primary insulin resistance: heterogeneity of molecular defects. Prog Clin Biol Res. 1982;97:245–281. [PubMed] [Google Scholar]
- Rogers D. R. Leprechaunism (Donohue's syndrome). A possible case, with emphasis on changes in the adenohypophysis. Am J Clin Pathol. 1966 May;45(5):614–619. doi: 10.1093/ajcp/45.5.614. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. M., Haworth J. C., Degroot G. W., Trevenen C. L., Rechler M. M. A case of leprechaunism with severe hyperinsulinemia. Am J Dis Child. 1980 Feb;134(2):170–175. doi: 10.1001/archpedi.1980.02130140044014. [DOI] [PubMed] [Google Scholar]
- Rüdiger H. W., Dreyer M., Kühnau J., Bartelheimer H. Familial insulin-resistant diabetes secondary to an affinity defect of the insulin receptor. Hum Genet. 1983;64(4):407–411. doi: 10.1007/BF00292378. [DOI] [PubMed] [Google Scholar]
- SALMON M. A., WEBB J. N. DYSTROPHIC CHANGES ASSOCIATED WITH LEPRECHAUNISM IN A MALE INFANT. Arch Dis Child. 1963 Oct;38:530–535. doi: 10.1136/adc.38.201.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi F., Maggiore G., Bianchi E., Marseglia G., Marconi M., Ugazio A. G., Siccardi A. G. Staphylocidal defect of polymorphonuclear leukocytes in a patient with leprechaunism. Eur J Pediatr. 1981 Sep;137(1):89–90. doi: 10.1007/BF00441177. [DOI] [PubMed] [Google Scholar]
- Santora A. C., 2nd, Wheeler F. B., DeHaan R. L., Elsas L. J., 2nd Relationship of insulin binding to amino acid transport by cultured 14-day embryonic chick heart cells. Endocrinology. 1979 Apr;104(4):1059–1068. doi: 10.1210/endo-104-4-1059. [DOI] [PubMed] [Google Scholar]
- Scarlett J. A., Kolterman O. G., Moore P., Saekow M., Insel J., Griffin J., Mako M., Rubenstein A. H., Olefsky J. M. Insulin resistance and diabetes due to a genetic defect in insulin receptors. J Clin Endocrinol Metab. 1982 Jul;55(1):123–131. doi: 10.1210/jcem-55-1-123. [DOI] [PubMed] [Google Scholar]
- Schilling E. E., Rechler M. M., Grunfeld C., Rosenberg A. M. Primary defect of insulin receptors in skin fibroblasts cultured from an infant with leprechaunism and insulin resistance. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5877–5881. doi: 10.1073/pnas.76.11.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serravezza J. C., Endo F., DeHaan R. L., Elsas L. J., 2nd Insulin-induced receptor loss reduces responsiveness of chick heart cells to insulin. Endocrinology. 1983 Aug;113(2):497–507. doi: 10.1210/endo-113-2-497. [DOI] [PubMed] [Google Scholar]
- Summitt R. L., Favara B. E. Leprechaunism (Donohue's syndrome): a case report. J Pediatr. 1969 Apr;74(4):601–610. doi: 10.1016/s0022-3476(69)80044-2. [DOI] [PubMed] [Google Scholar]
- Taylor S. I., Hedo J. A., Underhill L. H., Kasuga M., Elders M. J., Roth J. Extreme insulin resistance in association with abnormally high binding affinity of insulin receptors from a patient with leprechaunism: evidence for a defect intrinsic to the receptor. J Clin Endocrinol Metab. 1982 Dec;55(6):1108–1113. doi: 10.1210/jcem-55-6-1108. [DOI] [PubMed] [Google Scholar]
- Taylor S. I., Roth J., Blizzard R. M., Elders M. J. Qualitative abnormalities in insulin binding in a patient with extreme insulin resistance: decreased sensitivity to alterations in temperature and pH. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7157–7161. doi: 10.1073/pnas.78.11.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. I., Samuels B., Roth J., Kasuga M., Hedo J. A., Gorden P., Brasel D. E., Pokora T., Engel R. R. Decreased insulin binding in cultured lymphocytes from two patients with extreme insulin resistance. J Clin Endocrinol Metab. 1982 May;54(5):919–930. doi: 10.1210/jcem-54-5-919. [DOI] [PubMed] [Google Scholar]
- Tsujino G. A case of leprechaunism and an analysis of some clinical manifestations of this syndrome. Z Kinderheilkd. 1975;118(4):347–360. doi: 10.1007/BF00492338. [DOI] [PubMed] [Google Scholar]
- Verhoeven G. F., Wilson J. D. The syndromes of primary hormone resistance. Metabolism. 1979 Mar;28(3):253–289. doi: 10.1016/0026-0495(79)90072-6. [DOI] [PubMed] [Google Scholar]
- Wheeler F. B., Santora A. C., 2nd, Elsas L. J., 2nd Evidence supporting a two-receptor model for insulin binding by cultured embryonic heart cells. Endocrinology. 1980 Jul;107(1):195–207. doi: 10.1210/endo-107-1-195. [DOI] [PubMed] [Google Scholar]