Abstract
The immune system is equipped with an extremely large spectrum of structurally diverse receptors to recognize all potential antigens. This fundamental principle of receptor diversity is no longer upheld in patients with rheumatoid arthritis (RA), who have a marked contraction of the T cell receptor repertoire. In this study, the ability of RA patients to produce T cells and to maintain T cell homeostasis was examined. CD4 T cells containing T cell receptor rearrangement excision circles (TREC) were substantially reduced in RA patients; TREC levels in young adult patients matched those of controls 20 years older. Increased self-replication of T cells in RA was indicated by age-inappropriate erosion of telomeres in circulating T cells with almost complete attrition of telomeric reserves in patients 20–30 yr of age. The degree of telomere loss was not related to disease duration or the use of disease-modifying medication and was most pronounced in CD4+CD45ROnull (naive) T cells. The loss of TREC-positive T cells could be a consequence of a primary defect in peripheral T cell homeostasis. Alternatively, RA patients may have impaired thymic function with the increased turnover of peripheral T cells being a secondary compensatory event.
Rheumatoid arthritis (RA) is a chronic inflammatory disease that manifests in the synovial membrane and eventually causes irreversible destruction of tendons, cartilage, and bone. It has been suspected that the inflammatory lesions result from an autoimmune response to joint-specific antigens (1–3). Recent data, however, suggest that the immune abnormalities are not limited to the activation and clonal expansion of synovium-infiltrating lymphocytes, but involve the majority of circulating T cells. Analysis of the frequency of arbitrarily selected T cell receptors (TCR) derived from CD4 T cells demonstrated a sharp contraction in the diversity of T cells (4). In RA patients, the majority of T cells is clonally expanded. Some T cells have grown up to a large clonal size and have lost the expression of the CD28 molecule (5, 6). This contraction of TCR diversity is not restricted to CD4 memory T cells; it is even more prominent in the subset of naive CD4 T cells that lack previous antigenic experience. Diversity of naive T cells is generally maintained by the influx of new thymic emigrants (7). Loss of T cell diversity, oligoclonal proliferation, and emergence of CD28null T cells are typical features of the immune dysfunction in the elderly (8–12), which has in part been attributed to the demise of thymic function. The similarities in the abnormalities of the senescent and the RA immune system led us to hypothesize that RA patients have premature immunosenescence. Contraction of the repertoire of naive T cells, therefore, raises the question whether patients with RA have a defect in generating new T cells.
The current study has addressed whether mechanisms of T cell generation are intact and age-appropriate in patients with RA. Thymic output of T lymphocytes can be estimated by exploiting an intrinsic feature of the TCR rearrangement process (13–15). Episomal products of the TCR rearrangement, TCR rearrangement excision circles (TREC), can be detected in recent thymic emigrants populating the periphery. A decrease in influx of newly generated T cells will not only result in a decline of TREC-containing T cells, but will force the system to restore equilibrium by replicating available mature T cells (16, 17). The need for self-renewal imposes replicative stress on peripheral T cells that can be measured by determining the length of telomeric DNA repeats (18). Telomeres are highly specialized ends of chromosomes that have a direct role in DNA duplication and are shortened with cell proliferation. Therefore, consumption of telomeric DNA can be used to estimate the replicative history of cells in the T cell compartment.
Materials and Methods
Study Population.
This study was reviewed and approved by the Mayo Clinic Internal Review Board, and all study participants signed informed consent. Peripheral blood mononuclear cells (PBMC) were obtained from 51 patients who fulfilled the 1987 American College of Rheumatology criteria for the diagnosis of RA (13- to 80-yr-old) and 47 healthy controls (18- to 77-yr-old) (19). Clinical data were obtained by retrospective chart review. Disease duration ranged from 3 mo to 22 yr; 84% of the patients had a positive rheumatoid factor (RF). Control individuals did not have a personal or family history of inflammatory disease.
Purification of Lymphocyte Subsets.
PBMC were incubated with antibody-coated magnetic beads (Miltenyi Biotec, Auburn, CA), and CD4 and CD8 T cells were separated in a magnetic cell separator (VarioMACS, Miltenyi Biotec). In selected experiments, CD8 T cell-depleted PBMC were subsequently separated by using anti-CD45RO mAb-coated magnetic beads to enrich for CD4+CD45RO+ (memory) and CD4+CD45ROnull (naive) T cells. Purity of separated T cell subsets was 90–95%.
Telomere Length Analysis.
DNA was extracted from purified T cell subsets using the QIAamp Blood Kit (Qiagen, Chatsworth, CA) followed by the removal of excess salt (NAP-10 column, Amersham Pharmacia Biotech, Arlington Heights, IL). DNA (5 μg/20 μl) was digested with HinfI and RsaI (Roche Molecular Biochemicals–Boehringer Mannheim, Indianapolis, IN) overnight at 37°C and was separated on a 0.5% agarose gel. The gels were dried at 60°C for 20 min, denatured in 10× sodium hydroxide buffer, neutralized in 2× SSC buffer, and then prehybridized in 20× SSC buffer, 10× Denhardt's solution, and 0.1 M dibasic sodium phosphate for 2 h. The hybridization was performed with a telomere repeat-specific 32P-end-labeled (CCCTAA)3 probe at 37°C overnight. After five washings (0.5× SSC buffer), the gels were scanned with a PhosphorImager and analyzed (Molecular Analyst/Macintosh, Bio-Rad). Telomere terminal restriction fragment (TRF) length was calculated relative to a 33P-end-labeled HindIII ladder that was run in each gel. Results are given as the median TRF length.
Quantification of TREC.
Signal-joint TREC concentrations were determined by competitive PCR using primers and the internal standard recently described (14). PCR reactions were performed with 0.1 μg of DNA, 2.6 units of DNA polymerase (Expand High Fidelity PCR System, Roche Molecular Biochemicals–Boehringer Mannheim), 1.5 mM MgCl2, 0.2 mM dNTPs, and 0.6 μM of each primer in 25 μl under the following conditions: 95°C for 5 min followed by 95°C, 60°C, and 72°C for 30 sec each for 35 cycles. To each PCR reaction was added 104, 103, or 102 molecules of signal-joint standard (kindly provided by D. C. Douek, Dallas, TX). PCR products were separated on polyacrylamide gels, stained with SYBR-Gold Nucleic Acid Stain (Molecular Probes), and imaged and analyzed using storm 840 and imagequant software (Molecular Dynamics).
Cell Culture.
CD4+CD45ROnull and CD4+CD45RO+ T cells (1 × 106/ml) were stimulated with anti-CD3 mAb (10 ng/ml, OKT3, CRL 8001, American Type Culture Collection, Manassas, VA) and phorbol 12-myristate 13-acetate (0.5 ng/ml) and maintained in 20 units/ml IL-2 (Proleukin, Chiron, Emeryville, CA) at 2 × 105 to 1 × 106 cells/ml. At each stimulation, 1 × 105/ml of irradiated adherent cells of an unrelated donor were added. Cultures were restimulated with anti-CD3 mAb and phorbol 12-myristate 13-acetate every 10 days. Cell recovery was determined every 3 days, and the mean population doublings were calculated as ln(cell number at day n/cell number at day 0)/ln2.
Statistical Analysis.
The median TRF lengths and the number of TREC/μg DNA of CD4 T cells were correlated with age by regression analysis (sigmaplot, SPSS, Chicago). ANOVA was used to compare RA patients and control individuals. Multivariate regression analysis was used to analyze the contribution of age, gender, RF status, current or previous treatment with methotrexate and prednisone, and disease duration to the TRF length in RA patients. TRF length in T cell subsets of age-matched controls and RA patients were compared by t test (sigmastat, SPSS). Results of the in vitro mean population doublings in RA patients and age-matched controls were compared by one-way ANOVA (sigmastat, SPSS).
Results
Reduced Numbers of Recent Thymic Emigrants in Patients with RA.
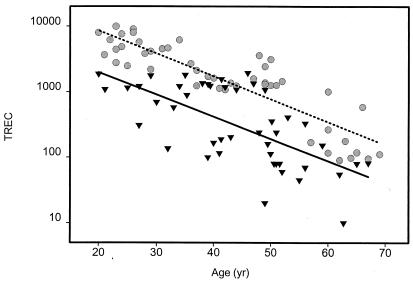
Thymic function of patients with RA was assessed by measuring the frequencies of TREC-containing CD4 T cells in the peripheral blood of RA patients and age-matched controls. In healthy individuals, the concentrations of TREC in CD4 T cells declined exponentially with age (Fig. 1). Between the ages of 20 and 70 yr, the numbers of TREC dropped by ≈1.5–2.0 logs with a slope of −0.035 TREC/yr (r2 = 0.77). TREC-containing CD4 T cells did not totally disappear in elderly individuals. Even in donors between 60 and 70 yr, >100 TREC/μg DNA were detected. RA patients had significantly lower levels of TREC in CD4 T cells than age-matched control donors (P < 0.0001). TREC concentrations were already markedly reduced in RA patients at the age of 20 yr; all of the 20- to 30-yr-old patients had values below the normal 10th percentile. The age-dependent decline in the number of TREC-containing CD4 T cells was not different (slope of −0.034 TREC/yr, r2 = 0.43) in the RA patients; however, the curves were shifted by approximately 20 yr toward a younger age. Only two of the control individuals had <90 TREC/μg DNA, and both donors were older than 60 yr of age. In contrast, TREC levels were <100 TREC/μg DNA in 26% of the patients.
Figure 1.
Decreased number of TREC-expressing CD4 T cells in RA patients. Signal joint TREC in peripheral CD4 T cells of control donors (circles) and RA patients (triangles) were quantified by competitive PCR as a marker of recent thymic emigrants. RA patients had a significantly lower number of TREC, suggesting reduced thymic activity. Regression lines of normals (dashed line) and patients (solid line) differed significantly (P = 0.0001). This difference was already apparent during early adulthood.
Increased Replicative History of Peripheral T Cells in RA.
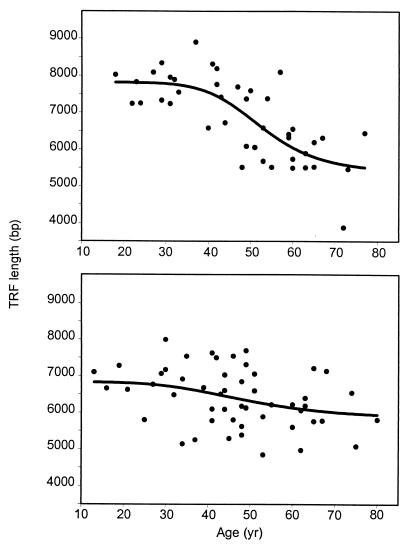
TREC concentrations are influenced by two parameters, the output of newly generated thymic T cells and the dilution of TREC-positive T cells through replication of peripheral T cells. The reduction of TREC in RA patients could indicate impaired thymic T cell production, which would induce compensatory growth of mature T cells. Age-inappropriate decline of TREC also could result from a primary dysregulation of peripheral T cell expansion. In either case, CD4 T cells of RA patients should have undergone increased replication. To provide an estimate of the replicative history, telomeric sequences of peripheral CD4 T cells were examined (Fig. 2). In normal individuals, TRF length declined progressively with age (r2 = 0.56, P = 0.0001). The rate of telomere erosion was not constant over a lifetime as assumed in previous studies (18, 20). The median TRF length remained essentially stable until age 40, entered a phase of accelerated reduction, and reached a new plateau at age 65. Between the ages of 40 and 65, control individuals lost an average of 76 bp/yr. In RA patients, TRF length was no longer significantly related to age (P = 0.5) and the distributions of TRF lengths in control donors and patients were different (P = 0.005). In the cohort <40 yr old, patients had an average TRF length of ≈6,300 bp, which was 1,400 bp shorter than in the age-matched control individuals. Between 40 and 65 yr of age, corresponding to the phase of accelerated telomere loss in controls, RA patients only experienced an average loss of 20 bp/yr. In the age group >65 yr old, patients and normal individuals were indistinguishable and reached the same plateau.
Figure 2.
Premature telomere erosion in CD4 cells of patients with RA. TRF length analysis was performed by using purified peripheral CD4 T cells of 42 control donors (Upper) and 51 RA patients (Lower). In control donors, TRF progressively shortened with age (r2 = 0.56, P = 0.0001), with an accelerated loss between the ages of 40 and 65 yr. In RA patients, TRF length did not correlate with age (r2 = 0.09, P = 0.5). Patients <40 yr already had nearly reached the plateau of aged control individuals (>65 yr). Regression lines for normals and patients differed significantly (P = 0.005).
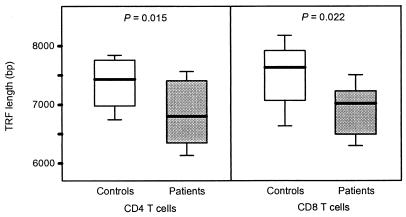
Erosion of telomeres in CD4 T cells was correlated with the decline in TREC, suggesting that the phenomena were mechanistically linked (r2 = 0.53, P = 0.05). Premature telomere erosion was not limited to CD4 T cells but also involved CD8 T cells. CD4 and CD8 T cells from PBMC of RA patients younger than 40 yr and age-matched controls were analyzed in parallel (Fig. 3). Telomeric sequences in both CD4 and CD8 T cell subsets were shortened significantly in the RA patients when compared to normal controls (P = 0.015 for CD4 T cells, P = 0.022 for CD8 T cells).
Figure 3.
Premature telomere shortening in RA patients affects CD4 and CD8 T cells. CD4 and CD8 T cells were purified from 16 RA patients and 14 control donors, all between the ages of 25 and 40 yr, and the TRF lengths were determined. Results are shown as box plots displaying medians, 25th and 75th percentiles as boxes, and 10th and 90th percentiles as whiskers. RA patients had significantly shorter telomeres in CD4 (P = 0.015) and CD8 T cells (P = 0.022).
Telomere Shortening of Naive T Cells in RA.
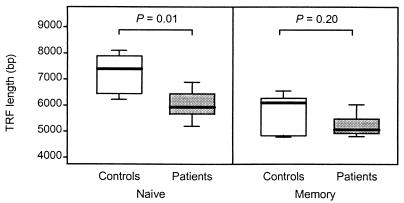
TREC-containing cells are found nearly exclusively in the compartment of naive T cells (21). However, the decline in TREC in RA patients is not associated with a decrease in size of the naive compartment; the frequency of CD45ROnull T cells in RA patients is similar to that in age-matched control donors (data not shown). To examine whether the increased telomere erosion involved the CD45ROnull T cells, TRF lengths were compared in memory and naive CD4 T cells from age-matched patients and control donors (Fig. 4). TRF lengths in naive CD4 T cells averaged 7.4 kb in the control individuals but were 1.5 kb shorter in the RA patients (P = 0.01). Consistent with their status of memory cells, CD4+CD45RO+ T cells had lost telomeric sequences (P = 0.004 for control donors, P = 0.02 for patients). Memory populations of control donors and patients differed by 1.0 kb, which did not approach statistical significance (P = 0.2). Thus, age-inappropriate erosion of telomeres in T lymphocytes of RA patients was mainly introduced by telomere shortening in naive T cells.
Figure 4.
Shortened telomeres in naive T cells from RA patients. CD4 T cells were separated into naive and memory T cells. Results from six RA patients and six age-matched controls are shown as box plots. Patients and control individuals differed in naive T cells (P = 0.01) but not in memory cells (P = 0.2).
Telomere Erosion and Clinical Variables.
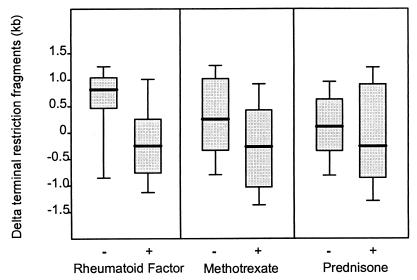
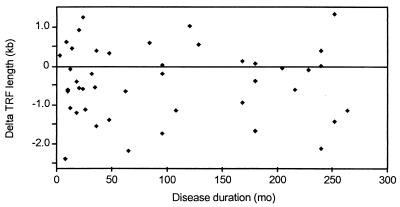
Multivariate analysis identified RF positivity as the only clinical variable correlating with telomere erosion in the RA cohort (P = 0.02). There was a trend for methotrexate-treated patients to have shorter telomeres (P = 0.06) but this group was biased for RF-positive patients with more severe disease and the trend was lost after correcting for RF positivity (P = 0.2). Exposure to corticosteroids had no apparent influence on telomere erosion (Fig. 5). Other disease-remitting agents were used too infrequently in the study population to have an impact. There also was no correlation between disease duration and TRF length (r2 = 0.0001, P = 0.44), suggesting that telomere erosion was not a sequela of the chronic inflammatory state. Disease duration ranged from 3 mo to 22 yr (Fig. 6). Telomere loss was present in the patients during the very early period of disease with no evidence for progressive shortening over time.
Figure 5.
Clinical correlative of telomere shortening. Results of telomere length analysis were evaluated by mutivariate regression analysis to determine the influence of clinical variables, including RF status and previous or current treatment. RF positivity showed a positive association that was independent of disease duration and age (P = 0.02). There was a trend for patients on methotrexate to have shorter telomeres (P = 0.06); however, this trend was lost after correction for RF-positivity. Data are expressed as the difference from the age-expected TRF and are shown as box plots.
Figure 6.
Telomere erosion and disease duration. TRF lengths in CD4 T cells from RA patients are expressed as the distance in kilobases from the age-appropriate point on the regression line for the normal population (Fig. 2). There was no correlation between the duration of RA and telomere loss. The results were unchanged when patients older than 50 yr were excluded from the analysis (data not shown).
Impaired Replicative Potential of CD4 T Cells in RA.
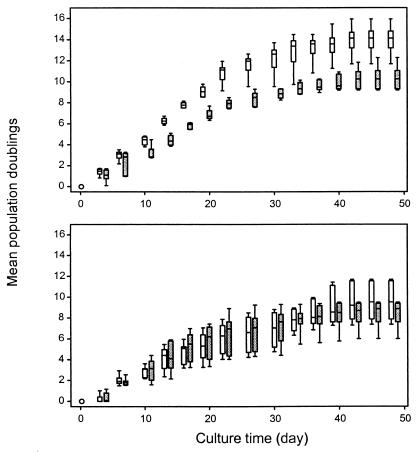
Replication and progressive telomeric erosion eventually limits the ability of a cell to divide. To explore whether the shortening of telomeric sequences in T cells of RA patients had functional consequences, the in vitro replicative capacity of CD4 T cells was determined. CD4 T cells were separated into CD45ROnull and CD45RO+ T cells, and T cell lines were established by polyclonal activation and maintained in IL-2. The mean population doublings were estimated in parallel samples from patients and age-matched control donors. Naive CD4 T cells from control individuals proliferated at a faster rate than naive T cells from RA patients (Fig. 7). Mean population doublings were 13.9 in the normal T cells compared to 10.3 in the RA T cells (P < 0.001). From these data, it was calculated that a naive T cell from an RA patient could be stimulated to grow to a clonal size of 1,261 cells. Under similar conditions, a naive T cell from a control individual would expand to 15,287 cells. Memory CD4 T cells from patients and control donors proliferated at similar rates (P = 0.95). In the memory populations, replicative senescence was reached after nine mean population doublings.
Figure 7.
Reduced replicative capacity in naive T cells from RA patients. Naive (Upper) and memory (Lower) T cells were purified from RA patients (shaded bars) and age-matched control donors (open bars) and stimulated in vitro. Cell recovery from parallel cultures was assessed every third day, and mean population doublings were calculated. Results are shown as box plots displaying medians, 25th and 75th percentiles as boxes, and 10th and 90th percentiles as whiskers. Growth rates and maximal clonal expansion were reduced in naive T cells from RA patients (P = 0.001).
Discussion
This study demonstrates that T cell dynamics in RA patients are fundamentally altered. Frequencies of TREC-expressing peripheral T cells, a measure for the output of newly generated T cells from the thymus, were age-inappropriately decreased with 20- to 30-yr-old patients having TREC levels equivalent to those of 50- to 60-yr-old healthy individuals. Lack of TREC-positive cells could result from acceleration of peripheral T cell turnover, possibly related to the disease process. However, abnormalities in T cell homeostasis were evident very early in disease with no progression over the course of the disease, suggesting a primary defect in T cell generation as an alternative explanation. In this model, a lack of thymic T cell production would impose proliferative demands on the peripheral T cells to fill the void and an increased self-replication of CD4+CD45ROnull T cells would lead to premature telomere erosion. Deficiency of T cell generation and the resulting abnormalities in maintaining a T cell pool need to be considered when designing therapies for RA patients.
Maintaining equilibrium in the T cell compartment is complex because the immune system is in constant turnover and the demands for lymphocyte replenishment are high (22, 23). T cells respond to antigenic challenge by clonal burst and mature effector cells are consumed in immune responses, in part by activation-induced apoptosis. The total size of the system is controlled, requiring a fine balance between influx of new T cells, efflux by consumption and death, and self-replication within the existing pool of lymphocytes. The thymus is critical in the generation of new T cells. With deficient influx, the system would have to respond by expanding mature T cells, leading to increased proliferative turnover and telomere shortening.
In the absence of phenotypic markers of recent thymic emigrants, measurements of TREC levels have been recently introduced as a valuable index of newly generated T cells entering the peripheral pool (13). TREC concentrations decrease with age, but TREC-expressing cells are not completely lost in the elderly. It is presently unclear how long TRECs persist in individual cells (24, 25). They are lost with cell division and a decline in TRECs could therefore be a reflection of increased peripheral turnover. Estimates of peripheral T cell turnover are not available in RA patients. Recent studies in healthy individuals and in HIV-infected patients using deuterium-labeled glucose have estimated the half-life of a peripheral T lymphocyte to be ≈100 days (26). Conversely, a recent study described that TRECs rapidly disappear after thymectomy in chickens with a half-life of only 14 days (15), suggesting that thymic activity is more important than peripheral turnover in determining TREC levels. The finding of TREC-positive cells in the elderly would then indicate some residual thymic function (21). Indeed, TREC concentrations can increase in adult patients with HIV infection on appropriate therapy (14).
Given that the number of TREC-containing CD4 T cells declines with cell division and that there are no data on the length of TREC persistence in mammalian cells, it is possible that increased T cell turnover and not defective T cell generation is the primary defect underlying the abnormalities in the RA patients. Ongoing antigenic stimulation in the inflammatory lesions should induce increased turnover of antigen-reactive cells, resulting in telomere erosion. In this model, memory cells would expand, compromise the size of the naive T cell compartment, and compete with the influx of new T cells and the survival of naive cells. Eventually, the entire T cell pool should be composed of antigen-expanded T cells at the expense of naive T cells. The invasion of the naive compartment by antigen-driven T cells would result in a loss of TREC-positive cells. The ability to generate immune responses to new antigens would be severely compromised and disease-relevant T cells would have replaced disease-unrelated T cells. To examine whether naive CD4+ T cells have been replaced by memory cells, we have used the CD45RO marker to distinguish between the T cell populations (27, 28). Compared to control individuals, CD4+CD45ROnull T cells are not decreased in RA patients. These data are consistent with the concept that the sizes of naive and memory compartments are independently controlled and suggest that the reduction in TREC-positive cells and telomere erosion does not necessarily reflect an expansion of the memory compartment (22). However, it has been shown in vivo and in vitro that memory T cells can resume the phenotype of a naive T cell, including the loss of the CD45RO molecule and the reexpression of CD45RA and CD62-ligand (29–33). It is unknown how frequently such phenotype conversions occur in vivo. Usefulness of the CD45RO marker in distinguishing T cell subsets with naive and memory characteristic is suggested by the finding that CD45RO+ and CD45ROnull T cells differ in TRF length and TCR diversity in RA patients and healthy individuals (4, 13, 18, 34).
Obviously, the two models of thymic dysfunction and high T cell turnover are not mutually exclusive. In fact, the observations in RA patients are reminiscent of the T cell dynamics in a murine experimental system. In this model, naive T cells, when resident in a T cell-depleted environment, started to proliferate vigorously to fill the space (16, 17). A memory phenotype was transiently acquired and many cells switched back to the naive phenotype. Proliferation of naive T cells can, however, occur without a change to the memory phenotype, as demonstrated in a minor lymphocyte-stimulating (MIs)-antigen driven system (35).
Disease-induced T cell proliferation should lead to progressive telomere loss as the disease process continues. In the current study, there was no correlation of telomere shortening with disease duration, arguing against a direct effect of chronic persistent RA on T cell homeostasis in the naive compartment. On the contrary, RA patients had a reduced number of TREC-expressing T cells even at age 20–25 yr. The slope of the age-dependent decline in TREC concentrations in RA patients was maintained compared to the control population, suggesting a normal turnover and an appropriate decline with advancing age and progressive disease duration. The curve was, however, shifted by ≈20 yr. Frequencies of TREC-positive cells are known to sharply decline between age 15 and 20 yr, corresponding to the time of thymic involution. One possible explanation is that thymic involution is accelerated in RA patients and occurs already at an earlier age. There is circumstantial evidence for a premature decline of lymphopoiesis in RA patients. Difficulties of RA patients to generate new T cells can be inferred from the results of clinical trials using T cell-depleting agents. RA patients treated with anti-T cell antibody frequently developed long-lasting lymphopenia, and the compartment of peripheral T cells contained large clonal populations (36, 37). Possible mechanisms of a premature thymic involution include age-inappropriate degeneration of thymic epithelium, required to support the differentiation of precursor cells into mature T cells (38). It also is possible that stem cells or thymocyte precursor cells are prematurely aged, impairing T cell generation (39).
Regardless of whether the reduced thymic output and the increased self-replication of peripheral lymphocytes in RA patients is an intrinsic feature of RA or a consequence of the autoimmune process, our data demonstrate that the loss of telomeres has functional consequences that may explain the increased susceptibility of RA patients to develop infections (40). Primary immune responses are characterized by a burst of T cell proliferation after antigenic stimulation and antigen-specific T cells expand to a large clonal size. Immunocompetence is critically determined by this ability to clonally expand. In terms of proliferation in response to TCR-triggering, naive T cells from RA patients were significantly impaired, with a maximal clonal expansion amounting to only one-tenth of that in control individuals.
These findings predict that the ability of RA patients to react to novel antigens, such as during infection, is compromised. Finally, the dysfunction of RA patients in reconstituting the T cell compartment should be considered when exploring new therapeutic strategies, particularly stem cell transplantation. Following bone marrow transplantation, RA patients might have major difficulties in recreating a diverse T cell pool, forcing them to use surviving memory T cells to rebuild the T cell compartment. If that were the case, the opportunity for reeducating the immune system, the underlying assumption in employing stem cell transplantation, could not be achieved without restoring the immune system's ability to generate new T cells.
Acknowledgments
We gratefully acknowledge Tammy J. Dahl and James W. Fulbright for editorial assistance and manuscript preparation and Cynthia Crowson for statistical analysis. This work was supported by the Mayo Foundation and National Institutes of Health Grants R01 AR41974, R01 AR42527, R21 GM58604, and 1 F05 TW05464–01.
Abbreviations
- PBMC
peripheral blood mononuclear cells
- RA
rheumatoid arthritis
- RF
rheumatoid factor
- TCR
T cell receptor
- TREC
T cell receptor rearrangement excision circles
- TRF
terminal restriction fragment
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Panayi G S. Curr Opin Rheumatol. 1997;9:236–240. doi: 10.1097/00002281-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Weyand C M, Goronzy J J. Med Clin N Am. 1997;81:29–55. doi: 10.1016/s0025-7125(05)70504-6. [DOI] [PubMed] [Google Scholar]
- 3.Nepom G T. Adv Immunol. 1998;68:315–332. doi: 10.1016/s0065-2776(08)60563-5. [DOI] [PubMed] [Google Scholar]
- 4.Wagner U G, Koetz K, Weyand C M, Goronzy J J. Proc Natl Acad Sci USA. 1998;95:14447–14452. doi: 10.1073/pnas.95.24.14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martens P B, Goronzy J J, Schaid D, Weyand C M. Arthritis Rheum. 1997;40:1106–1114. doi: 10.1002/art.1780400615. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt D, Goronzy J J, Weyand C M. J Clin Invest. 1996;97:2027–2037. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berzins S P, Godfrey D I, Miller J F, Boyd R L. Proc Natl Acad Sci USA. 1999;96:9787–9791. doi: 10.1073/pnas.96.17.9787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller R A. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 9.Posnett D N, Sinha R, Kabak S, Russo C. J Exp Med. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ku C C, Kotzin B, Kappler J, Marrack P. Immunol Rev. 1997;160:139–144. doi: 10.1111/j.1600-065x.1997.tb01034.x. [DOI] [PubMed] [Google Scholar]
- 11.Vallejo A N, Nestel A R, Schirmer M, Weyand C M, Goronzy J J. J Biol Chem. 1998;273:8119–8129. doi: 10.1074/jbc.273.14.8119. [DOI] [PubMed] [Google Scholar]
- 12.Weyand C M, Brandes J C, Schmidt D, Fulbright J W, Goronzy J J. Mech Ageing Dev. 1998;102:131–147. doi: 10.1016/s0047-6374(97)00161-9. [DOI] [PubMed] [Google Scholar]
- 13.Kong F, Chen C H, Cooper M D. Immunity. 1998;8:97–104. doi: 10.1016/s1074-7613(00)80462-8. [DOI] [PubMed] [Google Scholar]
- 14.Douek D C, McFarland R D, Keiser P H, Gage E A, Massey J M, Haynes B F, Polis M A, Haase A T, Feinberg M B, Sullivan J L, et al. Nature (London) 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 15.Kong F K, Chen C L, Six A, Hockett R D, Cooper M D. Proc Natl Acad Sci USA. 1999;96:1536–1540. doi: 10.1073/pnas.96.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ernst B, Lee D S, Chang J M, Sprent J, Surh C D. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 17.Goldrath A W, Bevan M J. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weng N P, Levine B L, June C H, Hodes R J. Proc Natl Acad Sci USA. 1995;92:11091–11094. doi: 10.1073/pnas.92.24.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S, Healey L A, Kaplan S R, Liang M H, Luthra H S. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 20.Ball S E, Gibson F M, Rizzo S, Tooze J A, Marsh J C, Gordon-Smith E C. Blood. 1998;91:3582–3592. [PubMed] [Google Scholar]
- 21.Poulin J F, Viswanathan M N, Harris J M, Komanduri K V, Wieder E, Ringuette N, Jenkins M, McCune J M, Sekaly R P. J Exp Med. 1999;190:479–486. doi: 10.1084/jem.190.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freitas A A, Rocha B. Curr Opin Immunol. 1999;11:152–156. doi: 10.1016/s0952-7915(99)80026-0. [DOI] [PubMed] [Google Scholar]
- 23.Tanchot C, Rosado M M, Agenes F, Freitas A A, Rocha B. Semin Immunol. 1997;9:331–337. doi: 10.1006/smim.1997.0090. [DOI] [PubMed] [Google Scholar]
- 24.Makino Y, Yamagata N, Sasho T, Adachi Y, Kanno R, Koseki H, Kanno M, Taniguchi M. J Exp Med. 1993;177:1399–1408. doi: 10.1084/jem.177.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak F, Schatz D G. Mol Cell Biol. 1996;16:609–618. doi: 10.1128/mcb.16.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellerstein M, Hanley M B, Cesar D, Siler S, Papageorgopoulos C, Wieder E, Schmidt D, Hoh R, Neese R, Macallan D, Deeks S, McCune J M. Nat Med. 1999;5:83–89. doi: 10.1038/4772. [DOI] [PubMed] [Google Scholar]
- 27.Sanders M E, Makgoba M W, Shaw S. Immunol Today. 1988;9:195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- 28.Dutton R W, Bradley L M, Swain S L. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 29.Arlettaz L, Barbey C, Dumont-Girard F, Helg C, Chapuis B, Roux E, Roosnek E. Eur J Immunol. 1999;29:3987–3994. doi: 10.1002/(SICI)1521-4141(199912)29:12<3987::AID-IMMU3987>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Bell E B, Sparshott S M, Bunce C. Immunol Today. 1998;19:60–64. doi: 10.1016/s0167-5699(97)01211-5. [DOI] [PubMed] [Google Scholar]
- 31.Bell E B, Sparshott S M. Nature (London) 1990;348:163–166. doi: 10.1038/348163a0. [DOI] [PubMed] [Google Scholar]
- 32.Michie C A, McLean A, Alcock C, Beverley P C. Nature (London) 1992;360:264–265. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- 33.Bunce C, Bell E B. J Exp Med. 1997;185:767–776. doi: 10.1084/jem.185.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arstila T P, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. Science. 1999;286:958–961. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- 35.Hayden K A, Tough D F, Webb S R. J Immunol. 1996;156:48–55. [PubMed] [Google Scholar]
- 36.Jendro M C, Ganten T, Matteson E L, Weyand C M, Goronzy J J. Arthritis Rheum. 1995;38:1242–1251. doi: 10.1002/art.1780380912. [DOI] [PubMed] [Google Scholar]
- 37.Moreland L W, Pratt P W, Mayes M D, Postlethwaite A, Weisman M H, Schnitzer T, Lightfoot R, Calabrese L, Zelinger D J, Woody J N. Arthritis Rheum. 1995;38:1581–1588. doi: 10.1002/art.1780381109. [DOI] [PubMed] [Google Scholar]
- 38.Fink P J, Bevan M J. Adv Immunol. 1995;59:99–133. doi: 10.1016/s0065-2776(08)60630-6. [DOI] [PubMed] [Google Scholar]
- 39.Shortman K, Wu L. Annu Rev Immunol. 1996;14:29–47. doi: 10.1146/annurev.immunol.14.1.29. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell D M, Spitz P W, Young D Y, Bloch D A, McShane D J, Fries J F. Arthritis Rheum. 1986;29:706–714. doi: 10.1002/art.1780290602. [DOI] [PubMed] [Google Scholar]