Abstract
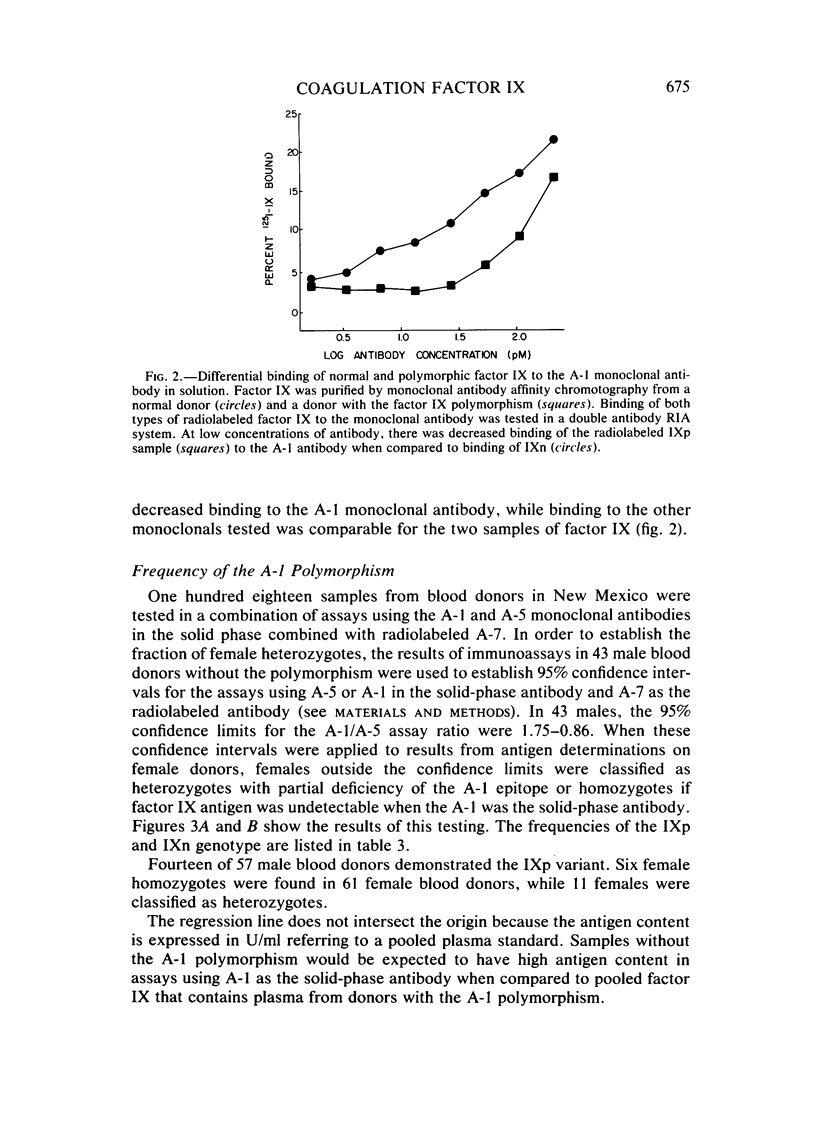
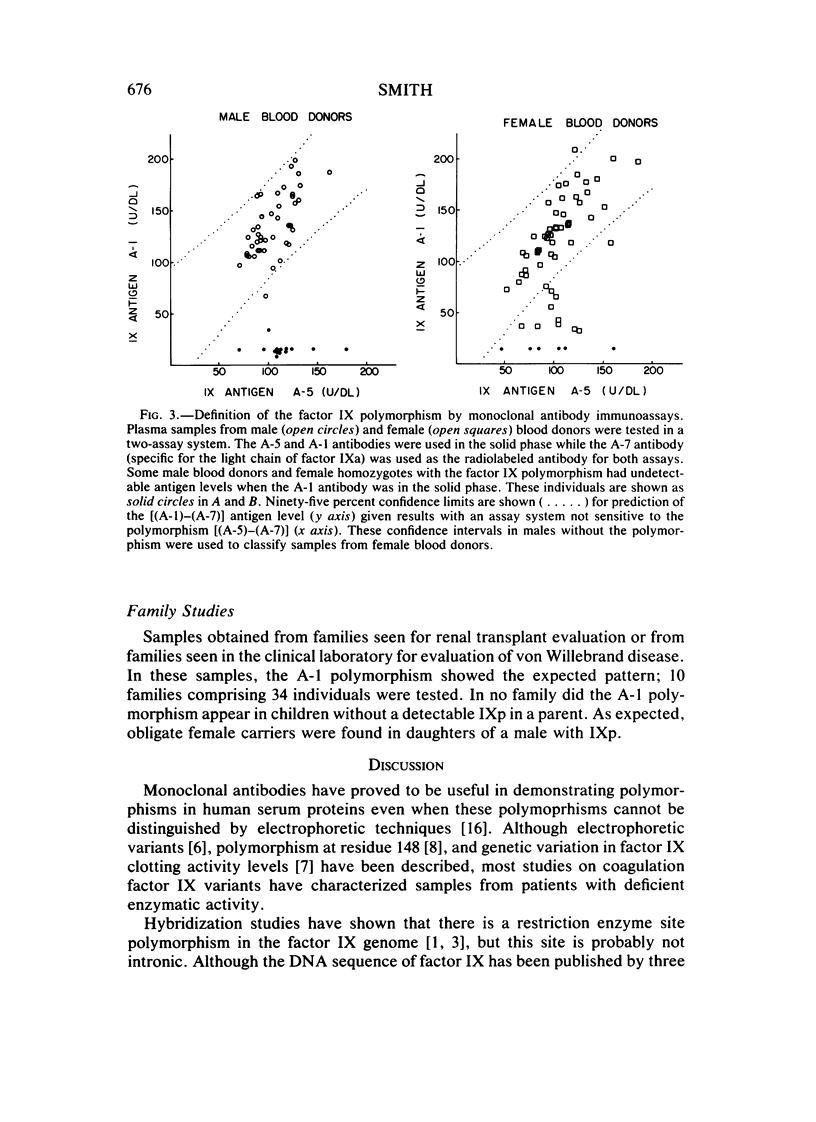
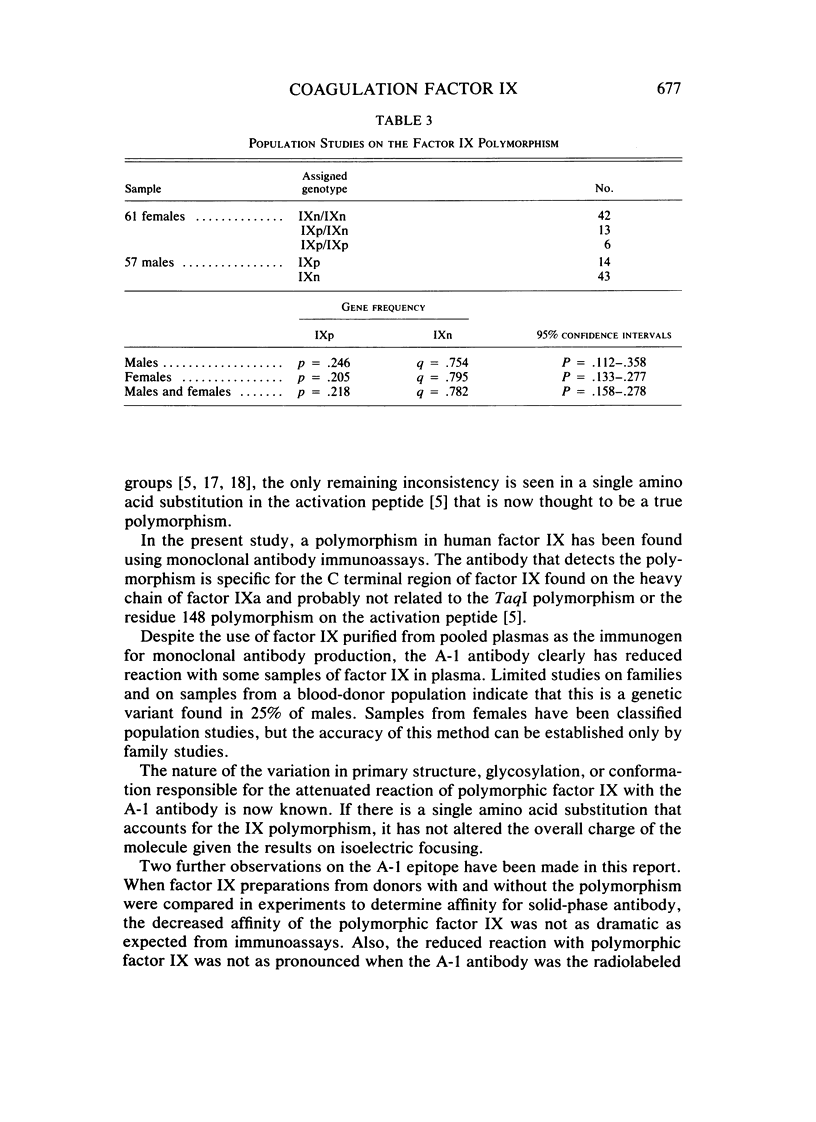
Monoclonal antibodies have been used to demonstrate a polymorphism of human plasma coagulation factor IX antigen in double antibody solid-phase immunoradiometric assays. This polymorphism is detected in an assay where a monoclonal antibody (A-1) adsorbed to microtiter wells is used to bind factor IX from diluted plasma samples. Plasma samples with the factor IX polymorphism have less than 0.2 U/ml of apparent antigen when tested with the A-1 antibody, while assays with other monoclonal antibodies and assays with goat antisera to factor IX show normal amounts of factor IX antigen. Factor IX coagulant activity was normal in samples from donors with the polymorphism. The thin-layer polyacrylamide gel isoelectric focusing pattern of factor IX purified from a donor with the factor IX polymorphism (IXp) was identical to that obtained with factor IX prepared from a donor who did not have the polymorphism (IXn). Purified radiolabeled factor IX prepared from a donor with the polymorphism showed a Ka for the A-1 antibody that was threefold less than that measured for IXn. The gene frequency of IXp in male blood donors is 0.25. This polymorphism may be useful as a marker for the X chromosome in genetic studies on plasma samples. Further studies are necessary to determine the explanation for decreased reaction of IXp with the A-1 monoclonal antibody.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anson D. S., Choo K. H., Rees D. J., Giannelli F., Gould K., Huddleston J. A., Brownlee G. G. The gene structure of human anti-haemophilic factor IX. EMBO J. 1984 May;3(5):1053–1060. doi: 10.1002/j.1460-2075.1984.tb01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd Y., Buckle V. J., Munro E. A., Choo K. H., Migeon B. R., Craig I. W. Assignment of the haemophilia B (factor IX) locus to the q26-qter region of the X chromosome. Ann Hum Genet. 1984 May;48(Pt 2):145–152. doi: 10.1111/j.1469-1809.1984.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Camerino G., Grzeschik K. H., Jaye M., De La Salle H., Tolstoshev P., Lecocq J. P., Heilig R., Mandel J. L. Regional localization on the human X chromosome and polymorphism of the coagulation factor IX gene (hemophilia B locus). Proc Natl Acad Sci U S A. 1984 Jan;81(2):498–502. doi: 10.1073/pnas.81.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannelli F., Anson D. S., Choo K. H., Rees D. J., Winship P. R., Ferrari N., Rizza C. R., Brownlee G. G. Characterisation and use of an intragenic polymorphic marker for detection of carriers of haemophilia B (factor IX deficiency). Lancet. 1984 Feb 4;1(8371):239–241. doi: 10.1016/s0140-6736(84)90122-3. [DOI] [PubMed] [Google Scholar]
- Grunebaum L., Cazenave J. P., Camerino G., Kloepfer C., Mandel J. L., Tolstoshev P., Jaye M., De la Salle H., Lecocq J. P. Carrier detection of Hemophilia B by using a restriction site polymorphism associated with the coagulation Factor IX gene. J Clin Invest. 1984 May;73(5):1491–1495. doi: 10.1172/JCI111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H. Applications of monoclonal antibodies in enzyme genetics. Annu Rev Genet. 1983;17:279–314. doi: 10.1146/annurev.ge.17.120183.001431. [DOI] [PubMed] [Google Scholar]
- Jaye M., de la Salle H., Schamber F., Balland A., Kohli V., Findeli A., Tolstoshev P., Lecocq J. P. Isolation of a human anti-haemophilic factor IX cDNA clone using a unique 52-base synthetic oligonucleotide probe deduced from the amino acid sequence of bovine factor IX. Nucleic Acids Res. 1983 Apr 25;11(8):2325–2335. doi: 10.1093/nar/11.8.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi K., Davie E. W. Isolation and characterization of a cDNA coding for human factor IX. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6461–6464. doi: 10.1073/pnas.79.21.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester R. H., Elston R. C., Graham J. B. Variations in levels of blood clotting factors IX and X in a population of normal men: possible genetic polymorphisms. Am J Hum Genet. 1972 Mar;24(2):168–180. [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Mizuochi T., Taniguchi T., Fujikawa K., Titani K., Kobata A. The structures of the carbohydrate moieties of bovine blood coagulation factor IX (Christmas factor). J Biol Chem. 1983 May 25;258(10):6020–6024. [PubMed] [Google Scholar]
- Smith K. J., Ono K. Monoclonal antibodies to factor IX: characterization and use in immunoassays for factor IX. Thromb Res. 1984 Jan 15;33(2):211–224. doi: 10.1016/0049-3848(84)90182-8. [DOI] [PubMed] [Google Scholar]
- Suomela H. Multiple forms of human factor IX in chromatography and isoelectric focusing. Thromb Res. 1975 Jul;7(1):101–112. doi: 10.1016/0049-3848(75)90128-0. [DOI] [PubMed] [Google Scholar]