Abstract
K562 is an established human erythroleukemia cell line, inducible for hemoglobin synthesis by a variety of compounds including n-butyrate. To elucidate the role of butyrate-induced histone acetylation in the regulation of gene expression in K562 cells, we isolated 20 variants resistant to the growth inhibitory effect of butyrate. Four variants having different degrees of resistance were selected for detailed study. All four were found to be resistant to the hemoglobin-inducing effect of butyrate, suggesting that the two aspects of butyrate response, restriction of growth and induction of hemoglobin synthesis, are coupled. Further, after (5 days) culture with butyrate, two of the four variants exhibit less acetylation of H3 and H4 histones than does the butyrate-treated parent. Analysis of histone deacetylases from the variants indicated that each variant was distinct and that butyrate resistance may be accounted for by decreased affinity of the variant enzymes for butyrate, increased affinity of the enzymes for acetylated histone, or both. The fact that variants selected for resistance to growth inhibition by butyrate are also deficient in butyrate-induced hemoglobin synthesis and have abnormal histone deacetylase activity argues for butyrate inducing K562 cells to synthesize hemoglobin and restrict growth via histone acetylation.
Full text
PDF



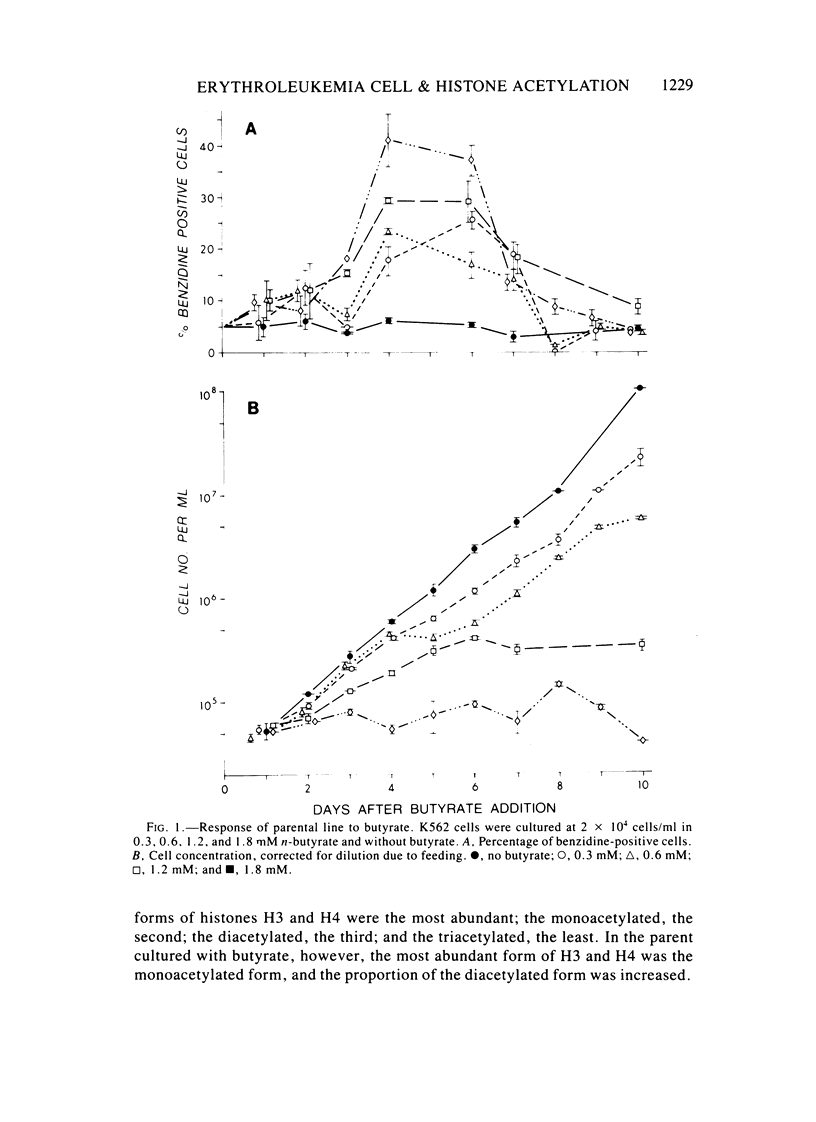
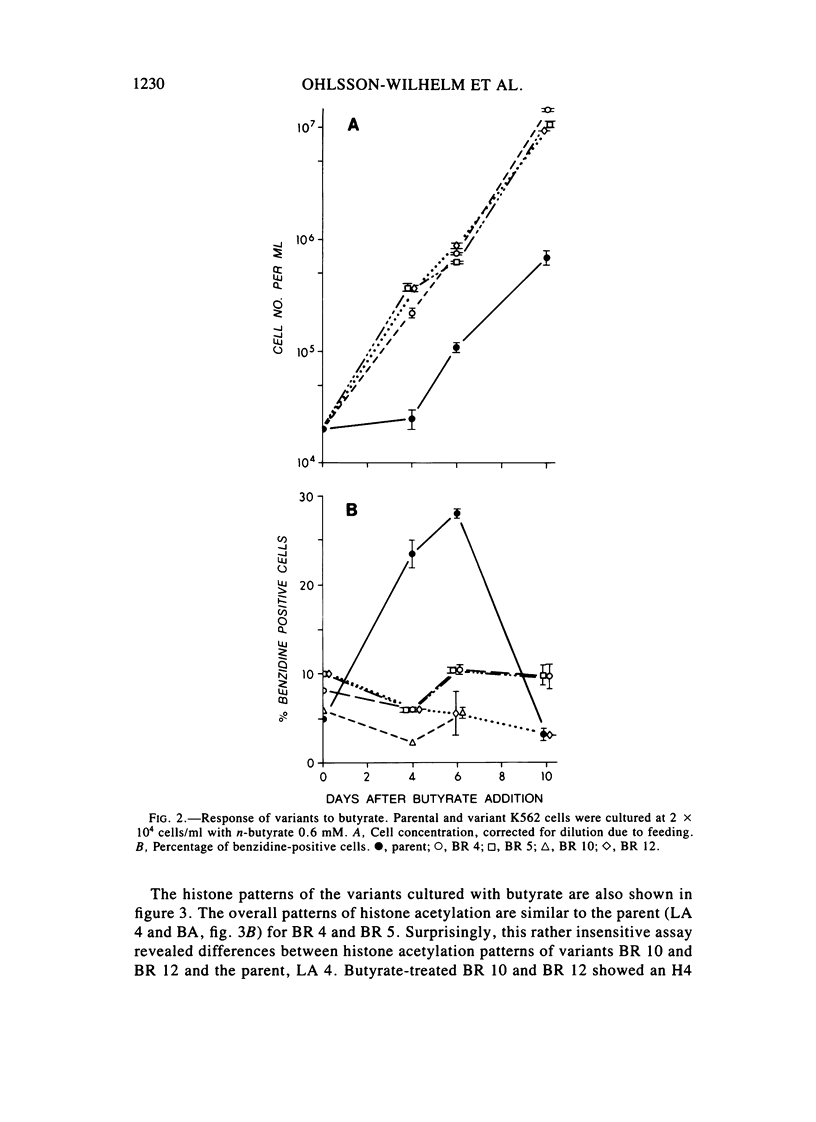
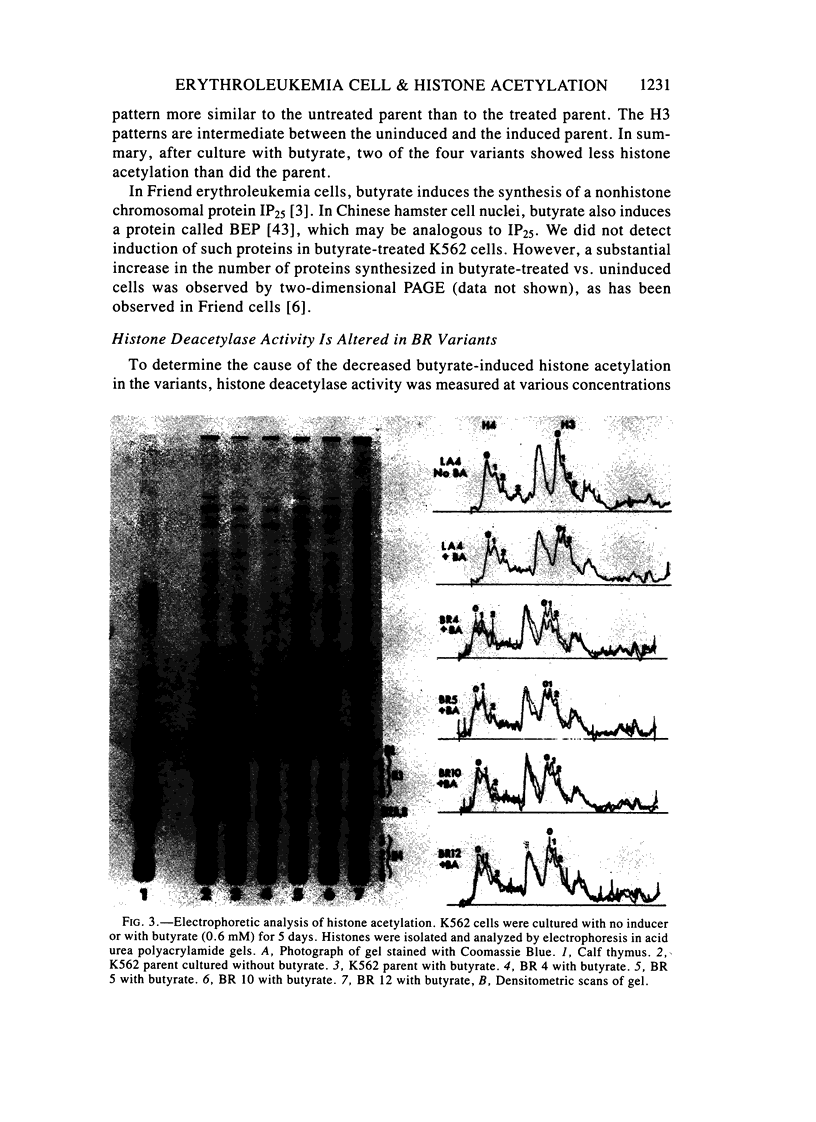





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLFREY V. G., FAULKNER R., MIRSKY A. E. ACETYLATION AND METHYLATION OF HISTONES AND THEIR POSSIBLE ROLE IN THE REGULATION OF RNA SYNTHESIS. Proc Natl Acad Sci U S A. 1964 May;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter B. P., Goff S. C., Efremov G. D., Gravely M. E., Huisman T. H. Globin chain electrophoresis: a new approach to the determination of the G gamma/A gamma ratio in fetal haemoglobin and to studies of globin synthesis. Br J Haematol. 1980 Apr;44(4):527–534. doi: 10.1111/j.1365-2141.1980.tb08706.x. [DOI] [PubMed] [Google Scholar]
- Andersson L. C., Jokinen M., Gahmberg C. G. Induction of erythroid differentiation in the human leukaemia cell line K562. Nature. 1979 Mar 22;278(5702):364–365. doi: 10.1038/278364a0. [DOI] [PubMed] [Google Scholar]
- Boffa L. C., Vidali G., Mann R. S., Allfrey V. G. Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. J Biol Chem. 1978 May 25;253(10):3364–3366. [PubMed] [Google Scholar]
- Candido E. P., Reeves R., Davie J. R. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978 May;14(1):105–113. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- Cioe L., McNab A., Hubbell H. R., Meo P., Curtis P., Rovera G. Differential expression of the globin genes in human leukemia K562(S) cells induced to differentiate by hemin or butyric acid. Cancer Res. 1981 Jan;41(1):237–243. [PubMed] [Google Scholar]
- Cousens L. S., Gallwitz D., Alberts B. M. Different accessibilities in chromatin to histone acetylase. J Biol Chem. 1979 Mar 10;254(5):1716–1723. [PubMed] [Google Scholar]
- D'Anna J. A., Tobey R. A., Gurley L. R. Concentration-dependent effects of sodium butyrate in Chinese hamster cells: cell-cycle progression, inner-histone acetylation, histone H1 dephosphorylation, and induction of an H1-like protein. Biochemistry. 1980 Jun 10;19(12):2656–2671. doi: 10.1021/bi00553a019. [DOI] [PubMed] [Google Scholar]
- Davie J. R., Candido E. P. Acetylated histone H4 is preferentially associated with template-active chromatin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3574–3577. doi: 10.1073/pnas.75.8.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie J. R., Saunders C. A., Walsh J. M., Weber S. C. Histone modifications in the yeast S. Cerevisiae. Nucleic Acids Res. 1981 Jul 10;9(13):3205–3216. doi: 10.1093/nar/9.13.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokhelar M. C., Testa U., Vainchenker W., Finale Y., Tetaud C., Salem P., Tursz T. NK cell sensitivity of the leukemic K 562 cells; effect of sodium butyrate and hemin induction. J Immunol. 1982 Jan;128(1):211–216. [PubMed] [Google Scholar]
- Gambari R., Raschellà G., Tripodi M., Farace M. G., Fantoni A. Desferrioxamine inhibits induced erythroid differentiation of human leukemic K-562 cells. Cell Differ. 1983 May;12(5):249–255. doi: 10.1016/0045-6039(83)90020-9. [DOI] [PubMed] [Google Scholar]
- Guerrasio A., Vainchenker W., Breton-Gorius J., Testa U., Rosa R., Thomopoulos P., Titeux M., Guichard J., Beuzard Y. Embryonic and fetal hemoglobin synthesis in K562 cell line. Blood Cells. 1981;7(1):165–176. [PubMed] [Google Scholar]
- Hagopian H. K., Riggs M. G., Swartz L. A., Ingram V. M. Effect of n-butyrate on DNA synthesis in chick fibroblasts and HeLa cells. Cell. 1977 Nov;12(3):855–860. doi: 10.1016/0092-8674(77)90284-7. [DOI] [PubMed] [Google Scholar]
- Hay C. W., Candido E. P. Histone deacetylase. Association with a nuclease resistant, high molecular weight fraction of HeLa cell chromatin. J Biol Chem. 1983 Mar 25;258(6):3726–3734. [PubMed] [Google Scholar]
- Hoffman R., Murnane M. J., Benz E. J., Jr, Prohaska R., Floyd V., Dainiak N., Forget B. G., Furthmayr H. Induction of erythropoietic colonies in a human chronic myelogenous leukemia cell line. Blood. 1979 Nov;54(5):1182–1187. [PubMed] [Google Scholar]
- Klein G., Zeuthen J., Eriksson I., Terasaki P., Bernoco M., Rosén A., Masucci G., Povey S., Ber R. Hybridization of a myeloid leukemia-derived human cell line (K562) with a human Burkitt's lymphoma line (P3HR-1). J Natl Cancer Inst. 1980 Apr;64(4):725–738. [PubMed] [Google Scholar]
- Kruh J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biochem. 1982 Feb 5;42(2):65–82. doi: 10.1007/BF00222695. [DOI] [PubMed] [Google Scholar]
- Leder A., Leder P. Butyric acid, a potent inducer of erythroid differentiation in cultured erythroleukemic cells. Cell. 1975 Jul;5(3):319–322. doi: 10.1016/0092-8674(75)90107-5. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Nelson D. A., Perry M., Sealy L., Chalkley R. DNAse I preferentially digests chromatin containing hyperacetylated histones. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1346–1353. doi: 10.1016/0006-291x(78)90337-6. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pantazis P., Bonner W. M. Butyrate-induced histone hyperacetylation in human and mouse cells: estimation of putative sites of histone acetylation in vivo. J Cell Biochem. 1982;20(3):225–235. doi: 10.1002/jcb.240200303. [DOI] [PubMed] [Google Scholar]
- Pantazis P., Lazarou S. A., Papadopoulos N. M. Isoenzymes of lactate dehydrogenase in human leukemic cells in culture treated with inducers of differentiation. J Cell Biol. 1981 Aug;90(2):396–401. doi: 10.1083/jcb.90.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Prasad K. N. Butyric acid: a small fatty acid with diverse biological functions. Life Sci. 1980 Oct 13;27(15):1351–1358. doi: 10.1016/0024-3205(80)90397-5. [DOI] [PubMed] [Google Scholar]
- Rastl E., Swetly P. Expression of poly(adenosine diphosphate-ribose) polymerase activity in erythroleukemic mouse cells during cell cycle and erythropoietic differentiation. J Biol Chem. 1978 Jun 25;253(12):4333–4340. [PubMed] [Google Scholar]
- Reeves R., Cserjesi P. Sodium butyrate induces new gene expression in Friend erythroleukemic cells. J Biol Chem. 1979 May 25;254(10):4283–4290. [PubMed] [Google Scholar]
- Riggs M. G., Whittaker R. G., Neumann J. R., Ingram V. M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977 Aug 4;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Rowley P. T., Ohlsson-Wilhelm B. M., Farley B. A., LaBella S. Inducers of erythroid differentiation in K562 human leukemia cells. Exp Hematol. 1981 Jan;9(1):32–37. [PubMed] [Google Scholar]
- Rowley P. T., Ohlsson-Wilhelm B. M., Rudolph N. S., Farley B. A., Kosciolek B., LaBella S. Hemin preferentially stimulates synthesis of alpha-globin in K562 human erythroleukemia cells. Blood. 1982 May;59(5):1098–1102. [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Wangh L. J., Allfrey V. G. Processing of newly synthesized histone molecules. Science. 1975 Oct 10;190(4210):117–128. doi: 10.1126/science.1166303. [DOI] [PubMed] [Google Scholar]
- Rutherford T. R., Clegg J. B., Weatherall D. J. K562 human leukaemic cells synthesise embryonic haemoglobin in response to haemin. Nature. 1979 Jul 12;280(5718):164–165. doi: 10.1038/280164a0. [DOI] [PubMed] [Google Scholar]
- Sanders L. A., Schechter N. M., McCarty K. S. A comparative study of histone acetylation, histone deacetylation, and ribonucleic acid synthesis in avian reticulocytes and erythrocytes. Biochemistry. 1973 Feb 27;12(5):783–791. doi: 10.1021/bi00729a001. [DOI] [PubMed] [Google Scholar]
- Sealy L., Chalkley R. The effect of sodium butyrate on histone modification. Cell. 1978 May;14(1):115–121. doi: 10.1016/0092-8674(78)90306-9. [DOI] [PubMed] [Google Scholar]
- Simpson R. T. Structure of chromatin containing extensively acetylated H3 and H4. Cell. 1978 Apr;13(4):691–699. doi: 10.1016/0092-8674(78)90219-2. [DOI] [PubMed] [Google Scholar]
- Testa U., Henri A., Bettaieb A., Titeux M., Vainchenker W., Tonthat H., Docklear M. C., Rochant H. Regulation of i- and I-antigen expression in the K562 cell line. Cancer Res. 1982 Nov;42(11):4694–4700. [PubMed] [Google Scholar]
- Testa U., Thomopoulos P., Vinci G., Titeux M., Bettaieb A., Vainchenker W., Rochant H. Transferrin binding to K562 cell line. Effect of heme and sodium butyrate induction. Exp Cell Res. 1982 Aug;140(2):251–260. doi: 10.1016/0014-4827(82)90112-4. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Baker R. M. Isolation of mutants of cultured mammalian cells. Methods Cell Biol. 1973;6:209–281. doi: 10.1016/s0091-679x(08)60052-7. [DOI] [PubMed] [Google Scholar]
- Vainchenker W., Testa U., Guichard J., Titeux M., Breton-Gorius J. Heterogeneity in the cellular commitment of a human leukemic cell line: K 562. Blood Cells. 1981;7(2):357–375. [PubMed] [Google Scholar]
- Vidali G., Boffa L. C., Bradbury E. M., Allfrey V. G. Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histones H3 and H4 and increased DNase I sensitivity of the associated DNA sequences. Proc Natl Acad Sci U S A. 1978 May;75(5):2239–2243. doi: 10.1073/pnas.75.5.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Wright J. A. Morphology and growth rate changes in Chinese hamster cells cultured in presence of sodium butyrate. Exp Cell Res. 1973 Apr;78(2):456–460. doi: 10.1016/0014-4827(73)90091-8. [DOI] [PubMed] [Google Scholar]