Abstract
In the human inflammatory myopathies (polymyositis and dermatomyositis), the early, widespread appearance of MHC class I on the surface of muscle cells and the occurrence of certain myositis-specific autoantibodies are striking features. We have used a controllable muscle-specific promoter system to up-regulate MHC class I in the skeletal muscles of young mice. These mice develop clinical, biochemical, histological, and immunological features very similar to human myositis. The disease is inflammatory, limited to skeletal muscles, self-sustaining, more severe in females, and often accompanied by autoantibodies, including, in some mice, autoantibodies to histidyl-tRNA synthetase, the most common specificity found in the spontaneous human disease, anti-Jo-1. This model suggests that an autoimmune disease may unfold in a highly specific pattern as the consequence of an apparently nonspecific event—the sustained up-regulation of MHC class I in a tissue—and that the specificity of the autoantibodies derives not from the specificity of the stimulus, but from the context, location, and probably the duration of the stimulus. This model further suggests that the presumed order of events as an autoimmune disease develops needs to be reconsidered.
In many autoimmune diseases, not only is the inflammatory destruction limited to particular target cells, but the associated autoantibodies are often highly disease specific. In myositis, for example, muscle cells are the target, and the autoantibodies against aminoacyl-tRNA synthetases, the signal recognition particle, or the Mi-2 nuclear protein (which are components of all cells) that occur in a significant proportion of cases are highly specific for myositis (1). In primary biliary cirrhosis, the inflammation affects intrahepatic biliary ducts, and the associated antimitochondrial autoantibodies, directed against the pyruvate dehydrogenase complex, occur in most cases and only rarely in other diseases (2). Such tight relationships naturally have fostered the view that a specific inciting agent is responsible for both the target cell injury and the autoantibodies. It has been generally assumed that the high specificity of target tissue damage and disease-specific autoantibodies derive from the structure of the inciting agent, but direct evidence for such a model has been sparse. Although much evidence suggests a role for inciting environmental agents as well as genetic factors in the development of autoimmune diseases, there are few, if any, persuasive examples connecting particular inciting agents to the later immunopathogenetic events (3), and although cross-reactivity with an inciting antigen would provide a convenient explanation for the breaking of tolerance, some carefully studied cases suggest that the endogenous target protein rather than a foreign antigen drives the autoantibody response (4).
The overexpression of MHC class I molecules is an early event in many autoimmune diseases, particularly in tissues which, like muscle, pancreatic β cells, and thyroid, have low or absent constitutive expression (5–7). The overexpression can occur in the absence of an inflammatory infiltrate, suggesting that it may be independent of and precede the effects of cytokines released from infiltrating mononuclear cells. Transgenic overexpression of MHC class I in several tissues has resulted in the destruction of the target tissue leading to insulin-dependent diabetes, a shivering phenotype with severe demyelination of the central nervous system, and Graves' disease in the absence of lymphocyte infiltration (8–10). In these mice, the transgenic MHC class I expression was driven by the respective tissue-specific promoters during the development of immune system in utero, with uncertain effects on tolerance. In a recently described mouse illness strongly resembling rheumatoid arthritis, the disease is genetically determined, both T and B cells are required, and an apparently driving antigen has been identified, but the reason that the inflammation is confined to the joints is not yet clear (11–13). Furthermore, in none of these models have the autoantibodies characteristic of the corresponding human disease been described. In another set of models of autoimmunity, such as knockout of transforming growth factor β (TGF-β) apoptosis inhibition, or graft-versus-host disease, generalized dysregulation of immunity leads to widespread inflammation and considerable nonspecific humoral autoimmunity (14–16).
In the human idiopathic inflammatory myopathies, the early, widespread appearance of MHC class I on the surface of muscle cells, even distant from lymphocytic infiltration, is a striking feature (17). Skeletal muscle cells do not constitutively express or display MHC class I molecules, although they can be induced to do so by proinflammatory cytokines such as IFN-γ and tumor necrosis factor α (18). The presence of proinflammatory cytokines in the affected tissue is inconstant (19, 20) and appears to be inadequate to account for the up-regulation. Because many events can up-regulate MHC class I on cell surfaces, we considered the possibility that the prolonged expression of MHC class I on the surface of muscle cells could itself be the inciting event of myositis, independent of a specific inciting stimulus. To avoid the possible effects of MHC class I on fetal development and present it to animals with a mature immune system, we used tetracycline regulation to control its expression.
Materials and Methods
Generation of Transgenic Mice.
The parent vector for the tetracycline-responsive element (TRE)-H-2Kb construct, pGEM H-2Kb, was a gift from J. Allison (Walter and Eliza Hall Institute of Medical Research, Victoria, Australia) (21). An XhoI-XbaI TRE fragment was released from the pTRE vector (CLONTECH) and digested with MspA1I to make a 3′ blunt end. The resulting 440-bp fragment was cloned into SalI-NruI sites of pGEM H-2Kb. The TRE-H-2Kb fragment (≈6.0 kb) was released by EcoRI-HindIII digest, gel-purified, and resuspended in the injection buffer (10 mmol/liter Tris⋅HCl, pH 7.4/0.1 mmol/liter EDTA). Founder mice (C57BL/6) carrying the TRE-H-2Kb transgene (H+) were identified by Southern hybridization analysis of tail DNA using a 440-bp TRE fragment as a probe (not shown). Generation of muscle-specific creatine kinase (MCK)-tTA mice (CBA/Ca X C57BL/6) was described (22). F6 MCK-tTA (backcrosses to C57BL/6) mice were crossed to H+ mice.
Animals transgenic for both a transactivator (tTA) under the control of a muscle creatine kinase promoter and the TRE-H-2Kb were detected by Southern analysis using both tTA and TRE probes. All H+ T+ mice were maintained either on s.c. implanted slow release (700 μg/day) doxycycline pellets (Innovative Research of America, Toledo, OH) or doxycycline hydrochloride (200 μg/ml) (Sigma) dissolved in 2% sucrose supplied as drinking water. Pellets with 21-day and 90-day release were used for the experiments. Doxycycline was withdrawn from some of the H+ T+ mice after 4 weeks whereas others were maintained on slow release doxycycline pellets for 1, 3, or 6 months. H+ T+ mice are fertile.
Total RNA from skeletal muscle was isolated by using Trizol reagent (Life Technologies, Grand Island, NY). The probe for hybridization was generated by PCR [H-2Kb-exon 3: 5′ tctctttcagtttggaggagt/5′ ccgcggcccctgcacctgtgcgca (366 bp)] and 32P-labeled by random priming. This probe detects both endogenous and transgenic H-2Kb. The membranes were hybridized overnight at 42°C in Hybrizol I (Intergen, Purchase, NY), washed, exposed to a PhosphorImager screen, and analyzed by a PhosphorImaging system (Molecular Dynamics).
The following probes were used for Northern blot analysis of skeletal muscle mRNA: endogenous H-2Kb-specific probe, an ≈600-bp fragment upstream from the ATG of the H-2Kb gene which is not present in the transgenic H-2Kb, detects only endogenous H-2Kb message. The probe for intercellular adhesion molecule-1 (ICAM-1) was generated by PCR with primer pairs 5′ tccgtctgcaggtcatcttagg/5′gtcgaaggtggttcttctgagc (292 bp) and β-actin probe (CLONTECH) was used as RNA loading control. The RNase protection assay was performed by using custom-made cytokine multiprobe template sets (set 1: granulocyte/macrophage colony-stimulating factor, IL-15, TGF-β1; set 2: tumor necrosis factor α, IL-1α, IL-1β, TGF-β1, and IL-6) and a commercial chemokine multiprobe template set [mCK-5: lymphotactin (Ltn), RANTES, Eotaxin, macrophage inflammatory protein-1β (MIP-1β), MIP-1α, MIP-2, IP-10, monocyte chemotactic protein-1 (MCP-1), T cell activation gene-3 (TCA-3) according to the manufacturer's instructions (PharMingen). Housekeeping genes L32 and glyceraldehyde-3-phosphate dehydrogenase were included in each set.
Phenotype of Transgenic Mice.
Locomotor activity was tested in pairs of mice at 3.5 months after the induction of transgene in an open field Digiscan apparatus (Omnitech Electronics, Columbus, OH); total distance and vertical activity were recorded every 10 min for a 1-h session. Ten pairs were monitored 6–8 times over a period of 2 weeks, and the results were calculated as means ± SE of all recordings. Serum creatine phosphokinase (CK) and glutamic-oxaloacetic transaminase levels (n = 8 per group) were measured on fresh serum samples by using a Hitachi 917 analyzer and reagents from Boehringer Mannheim. Statistical analyses were performed by using the one-way ANOVA test and t test with the sigmaplot 3.0 program. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Immunohistochemistry.
Tissues were snap-frozen in isopentane chilled in liquid nitrogen, cut in 8-μm sections, and processed for immunohistochemistry. Sections were incubated with 10 μg/ml FITC-conjugated anti-H-2Kb mAb (PharMingen, clone AF6–88.5) followed by rabbit anti-FITC antibody and then goat anti-rabbit-horseradish peroxidase (Dako). The slides were developed by diaminobenzidine substrate and tissues were counterstained briefly with hematoxylin before mounting. We used monocyte macrophage-2 (MOMA-2) antibody to detect cells of the monocyte/macrophage lineage. Anti-ICAM-1 and anti-I-Ab antibodies (PharMingen) were used to determine the activation status of the infiltrated macrophages.
Immunofluorescence and ELISA for Autoantibodies.
For the detection of autoantibodies, test serum samples along with positive and negative controls at 1:40 dilution were incubated for 2 h at room temperature with Hep2 cells according to standard clinical procedures. The slides then were incubated for another 2 h with FITC-conjugated anti-mouse IgG (Fc specific: 1:20 dilution). The slides were rinsed, mounted, and observed by fluorescence microscopy. To detect antibodies to histidyl-tRNA synthetase (HRS), we used an ELISA assay in which HPLC-purified, recombinant human HRS was the antigen (23). Statistical analyses were performed by t test with the sigmaplot 3.0 program.
Results
Controlled Up-Regulation of MHC Class I.
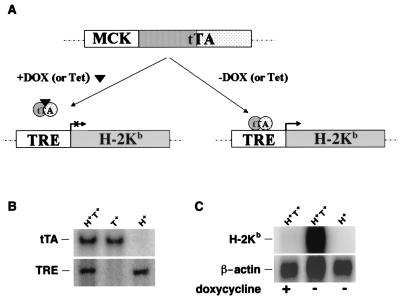
To develop a model in which we could control both the time and location of the expression of MHC class I, we generated founder mice (C57BL/6) that have TRE-H-2Kb stably integrated in the germ line (Fig. 1A). These target mice were crossed with mice that stably express the tetracycline-controlled tTA in skeletal muscle by means of a MCK promoter (MCK-tTA) (22). The inducible system is operable only in mice having both transgenes [TRE-H-2Kb (H+)/MCK-tTA (T+)]. In H+ T+ mice, the binding of doxycycline, a tetracycline analog, to tTA prevents the transactivator from binding to the TRE region, thereby preventing target gene expression (Fig. 1A). Thus, the transgenic H-2Kb expression can be induced by removing doxycycline and suppressed by administering it.
Figure 1.
(A) Schematic outline of the tetracycline regulatory system showing the MCK-tTA and TRE-H-2Kb transgenes. (B) Southern analysis of transgenic mice by using both tTA and TRE probes. (C) Northern analysis of mRNA from skeletal muscle of H+ T+ and H+ mice with (+) or without (−) doxycycline administration.
When the H-2Kb transgene was allowed to express in utero, no H+ T+ mice were identified among the progeny. However, H+ T+ mice were identified among live 18-day embryos, suggesting that the H-2Kb expression in skeletal muscle may be associated with parturition problems. In fact, several mummified H+ T+ fetuses were found retained in the uterus. The finding was not surprising because it had been previously shown that overexpression of the H-2Kb transgene under the control of a housekeeping promoter resulted in fetal loss (24). In the experiments reported here, all female single transgenic mice used for breeding were administered slow-releasing s.c. doxycycline pellets to suppress H-2Kb expression in the pups in utero. Under these conditions, H+ T+ mice were born at the expected Mendelian ratio. After weaning, all of the pups were maintained on doxycycline water until genotyping by Southern blotting at 4 weeks of age (Fig. 1B). Single transgenic pups born to the doxycycline-administered mothers were phenotypically and histologically normal. Single transgenic mice showed normal growth and are fertile. After genotyping, doxycycline water was removed from some H+ T+ mice to allow the induction of the H-2Kb gene expression.
In H+ T+ mice, removing doxycycline led to skeletal muscle-specific H-2Kb expression. Northern blot analysis of muscle RNA showed very high levels of the transgene mRNA in the absence of doxycycline and almost no detectable H-2Kb expression in the presence of doxycycline, indicating that the H-2Kb transgene is externally regulated. No detectable endogenous H-2Kb expression was seen in the skeletal muscle of the wild-type control or single transgenic mice (H+ or T+) in the absence of doxycycline (Fig. 1C).
Clinical, Biochemical, and Pathological Changes.
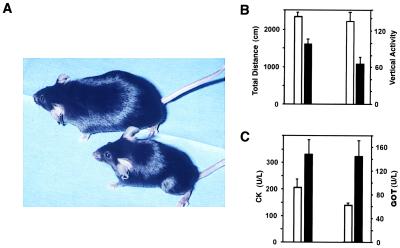
Female H+ T+ mice developed clinical signs of muscle weakness at about 3 months of age (2 months after transgene induction). The symptoms, which included arched spine, as well as lower back and hind limb weakness and wasting, become severe by 5–7 months of age (Fig. 2A). At 6 months of age, female H+ T+ mice had a 40–50% reduction in body weight compared with the sex-matched H+ or T+ transgenic littermates. Significantly reduced locomotor activity in an open field was registered as early as 1 month after doxycycline removal and was striking by 5–7 months of age (Fig. 2B). It was accompanied by increased serum levels of creatine kinase and glutamic-oxaloacetic transaminase, indicating ongoing muscle parenchymal damage (Fig. 2C). Interestingly, male H+ T+ mice remained free of clinical symptoms and other abnormalities up to 5 months of age, but they all developed clinical symptoms after 7 months of age. No rashes were seen in any H+ T+ mice.
Figure 2.
(A) Clinical phenotype of an H+ T+ female mouse at 5 months of age (Lower) showing significantly smaller size and lower back muscle wasting compared with a female H+ littermate (Upper). (B) Locomotor activity in pairs of mice at 3.5 months after the induction of the transgene. Total distance P < 0.001; vertical activity P < 0.0001. (C) Serum creatine phosphokinase (CK), P < 0.01; and glutamic-oxaloacetic transaminase (GOT) levels P < 0.01 (n = 8 per group); H+ T+ (filled bars) and control H+ or T+ female littermates (empty bars).
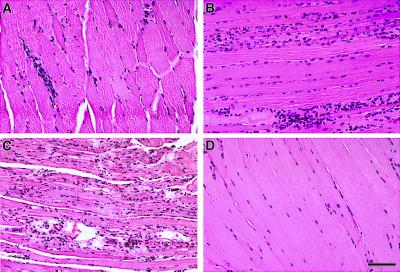
In the initial stages of gene expression (age 1.5 months), most of the muscle fibers remained normal (Fig. 3A). By 3.5 months, the skeletal muscles were distinctly abnormal with centralized nuclei, muscle fiber degeneration, and macrophage infiltration (Fig. 3B), and by 5.5 months, there was profound structural damage (Fig. 3C). The skeletal muscles of the H+ T+ mice at 5.5 months showed centralized nuclei, significant variation in muscle fiber diameter, muscle fiber degeneration and regeneration, atrophy, obliteration of the striations of the contractile apparatus, mononuclear cell infiltration, and the invasion of some muscle fibers by phagocytes (Fig. 3C). The skeletal muscle from 5.5-month-old single transgenic (T+ or H+) mice was unremarkable (Fig. 3D).
Figure 3.
Hematoxylin-eosin staining of formalin-fixed sections at different stages of the disease. (A) 1.5 months of age H+ T+ mice; (B) 3.5 months of age H+ T+ mice; (C) 5.5 months of age H+ T+ mice; and (D) 5.5 months of age, single transgenic T+ littermate. (Scale bar: A–D, 100 μm.)
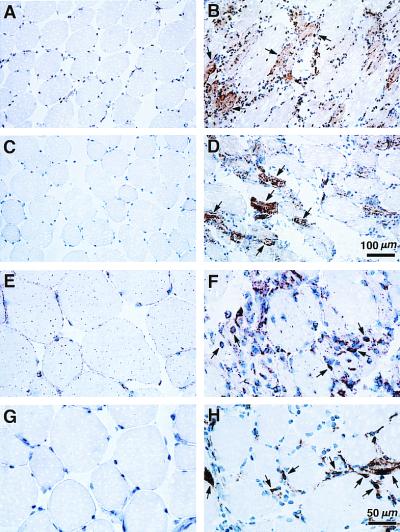
Immunohistochemical studies revealed reactivity for H-2Kb in many muscle fibers of H+ T+ mice. Staining was particularly strong in degenerating and regenerating muscle fibers (Fig. 4B). Several muscle fibers also showed strong reactivity to anti-ICAM-1 antibody (Fig. 4D), indicating the activation of other endogenous genes, typically up-regulated during muscle inflammation. By 5.5 months, the infiltrate was mostly of the macrophage/monocyte lineage (Fig. 4F). The infiltrating mononuclear cells also strongly stained positive for I-Ab (Fig. 4H) and ICAM-1 (data not shown), suggesting some of these cells may be of dendritic cell lineage. Muscle tissues from single transgenic littermates showed no reactivity for H-2Kb, I-Ab, and ICAM-1. Occasional CD3+ T cells (less than 1%) were present on immunohistochemical analysis, but heavy lymphocyte infiltration was not observed. No abnormalities were found in the heart, liver, kidney, lung, skin, or spleen. Mice carrying either transgene alone showed no pathologic abnormalities. In particular, no muscle fiber degeneration was observed in T+ mice, indicating that tTA expression in muscle was not responsible for the changes (Fig. 3D). The data suggest that the inflammatory changes in the H+ T+ mice were accompanied by the appearance in the affected muscles of other molecules typically found to be up-regulated in the human disease (20, 25). An increased level of expression of endogenous H-2Kb, and ICAM-1 on muscle fibers accompanied the up-regulation of the transgenic H-2Kb (Fig. 6C, lane 2). Furthermore, mRNAs for the chemokines MIP-1α and MCP-1 and the cytokine IL-15 were detected by the RNase protection assay in the H+ T+ mice but not in H+ or T+ controls (data not shown), strongly suggesting that chronic up-regulation of MHC class I triggers a cascade of events typical of muscle inflammation in the spontaneous human disease. Thus, H-2Kb expression alone, in the absence of significant T-lymphocyte infiltration, led to a sustained myopathy.
Figure 4.
Immunohistological findings in 5.5-month-old H+ T+ female (Right) and T+ female littermate (Left). Immunohistochemistry for H-2Kb (A and B), ICAM-1 (C and D), MOMA-2 for monocyte/macrophage lineage cells (E and F), and I-Ab (G and H). No reactivity was found for the above antibodies in single transgenic control mice except for the occasional presence of I-Ab positive mononuclear cells (data not shown). [Scale bars and magnifications: (A–D) 100 μm and ×100 and (E–H) 50 μm and ×200.]
Figure 6.
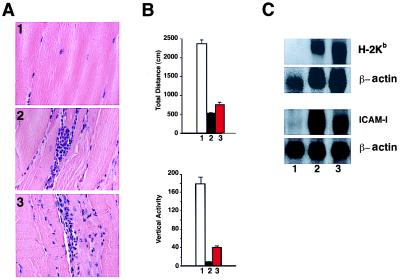
The readministration of doxycycline to H+ T+ mice manifesting disease. (A) Hematoxylin-eosin staining of skeletal muscle; (1) T+ mouse at 9 months of age; (2) H+ T+ mouse without doxycycline at 9 months of age; and (3) H+ T+ mouse retreated with doxycycline for 5 months after 4 months without doxycycline. (B) Locomotor activity (total distance and vertical activity); lane 1, T+ mice (white bar); lanes 2 and 3, H+ T+ mice without doxycycline (black bar); and H+ T+ mice retreated with doxycycline for 5 months after 4 months of disease (red bar). (C) Northern blot analysis of skeletal muscle mRNA for endogenous H-2Kb and ICAM-1. Control T+ (lane 1) and H+ T+ mice before and after readministration of doxycycline (lanes 2 and 3).
Humoral Autoimmunity.
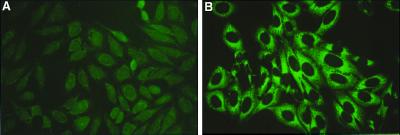
We sought evidence of humoral autoimmunity in the H+ T+ mice. The induction of H-2Kb expression resulted in detectable levels of antinuclear antibodies by immunofluorescence in 5 of 18 H+ T+ mice, and in 0 of 5 sex-matched littermates. More strikingly, however, the sera of some H+ T+ mice caused cytoplasmic staining condensed around the nucleus, which diminished toward the periphery of the cell (Fig. 5). This pattern is indistinguishable from the pattern of immunofluorescent cytoplasmic staining observed in the sera from patients with known autoantibodies to HRS or other anti-aminoacyl-tRNA synthetases. By using an ELISA assay in which purified recombinant human HRS was the antigen (23), elevated levels of autoantibodies to this known myositis-specific target autoantigen were detected in 8 of 23 H+ T+ mice, including both of the mice whose sera gave the fine cytoplasmic immunofluorescent pattern, but in none of 20 controls. Significantly elevated levels were observed in some double transgenic mice. In a comparison of anti-HRS titers tested at a 1:100 dilution, there was no difference between control mice (single transgenic H+ or T+ or nontransgenic) and double transgenic anti-HRS ELISA-negative mice (0.25 ± 0.02 vs. 0.27 ± 0.03; P = ns), but double transgenic anti-HRS ELISA-positive mice were clearly higher (0.25 ± 0.02 vs. 0.6 ± 0.24; P < 0.0001). We further confirmed the specificity of the ELISA-positive antibodies by Western blotting of sera against the purified human recombinant HRS antigen. Sera from ELISA-positive mice but not from control mice reacted with a ≈55-kDa band corresponding to the purified HRS (data not shown). The levels of serum muscle enzymes (creatine phosphokinase or glutamic-oxaloacetic transaminase) did not correlate with the anti-HRS antibody titers.
Figure 5.
Immunofluorescent staining on Hep2 cells to detect autoantibodies. Sera from a T+ female mouse (A) and an H+ T+ female mouse (B). The H+ T+ serum shows fine cytoplasmic staining condensed around the nucleus, which diminishes toward the periphery.
Failure to Reverse Disease.
In an attempt to down-regulate expression of the transgene, we readministered doxycycline to H+ T+ mice at 4 months of age, after the establishment of clinical, pathological, and biochemical disease and kept them on the drug for the next 5 months. There was negligible histological or clinical improvement (Fig. 6 A and B), and the level of endogenous H-2Kb expression remained high, as did the expression of ICAM-1 (Fig. 6C, lane 3). We also looked for various cytokines and chemokines that are up-regulated in human myositis biopsies. We found that MIP1-α, MCP-1, and IL-15 are specifically detected in the double transgenic mice. TGF-β1, TGF-β2, IL-6, MIP-1β, and IP-10 were detected in both single and double transgenic mice. We could not detect granulocyte/macrophage colony-stimulating factor, tumor necrosis factor type α (TNF-α), IL-1α, IL-1β, Ltn, RANTES, Eotaxin, MIP-2, or TCA-3 in the muscle tissues of either single or double transgenic mice. The up-regulated endogenous molecules and the proinflammatory chemokines and cytokines synthesized in the milieu presumably sustained the disease process.
Thus, the overexpression of MHC class I molecules alone in skeletal muscle led to a self-sustaining inflammatory process involving muscle fiber damage and infiltration of mononuclear cells with most of the characteristic features of the human idiopathic inflammatory myopathy, polymyositis, including the development of myositis-specific autoantibodies.
Discussion
Because a major immunological role of MHC molecules is the presentation of antigens to the immune system, it might be expected that they could have an important role in provoking autoimmune diseases. Each tissue or even each cell type is likely to have its own way of defending itself against autoimmune attack. Muscle, brain, eye, testes, and even islets, with their limited regenerative power, may use pathways that are especially suited to their circumstances (26). For example, it is known that muscle, besides expressing little or no MHC class I, constitutively expresses TGF-β, and that myocytes are very resistant to apoptosis (27–31), apparently through the up-regulation of antiapoptotic molecules when under an apoptotic threat (32). Although it is possible that the initiation of muscle fiber damage seen in this model results from other deleterious effects of the aberrant expression of MHC class I, as was considered in the earlier observations on pancreatic β cells, oligodendrocytes, and thyrocytes (8–10), the events that follow clearly reflect an immunological process.
The route leading from the expression of the transgenic H-2Kb to the anti-HRS autoantibodies remains a matter for speculation, raising the same questions common to all discussion of the breaking of tolerance in the periphery. The conditions we observed in the H+ T+ mice with up-regulated H-2Kb would allow several of the proposed mechanisms to operate. Human skeletal muscle myoblasts stimulated by cytokines to express MHC class I, class II, and ICAM-1 can effectively present antigens to autologous, antigen-specific CD4+ T cell lines (33); muscle cells can transfer antigens to professional antigen-presenting cells (34); and activated professional antigen-presenting cells can themselves take up endogenous antigens by endocytosis or pinocytosis for presentation. In the affected muscles of H+ T+ mice, muscle cells expressing MHC class I and ICAM-1 and mononuclear phagocytes expressing MOMA2+, and MHC class II+ are present in close proximity. The reasons why a ubiquitous antigen such as HRS is particularly the target of a humoral autoimmune response in humans with myositis and in these mice are not clear, but it is possible that HRS liberated from degenerating muscle cells contributes additional chemokine or cytokine activity, such as has been described for human tyrosyl-tRNA synthetase (35).
Because all H+ T+ animals induced to express the H-2Kb transgene develop clinical illness, but not all have measurable anti-HRS, it is unlikely that the antibodies have a direct pathogenic role. In general, there is little evidence that autoantibodies against obligatory intracellular components have such a role, except in those unusual cases in which the antigen can be shown to appear on cell surfaces (36). It is of considerable interest that in both dermatomyositis, an illness in which vascular damage and inflammation are dominant, and polymyositis, an illness in which CD8+ lymphocyte-induced cytotoxic injury to muscle cells is dominant (17, 37–42), anti-HRS antibodies can occur, suggesting that some common event (for example, the up-regulation of MHC class I) is responsible, as in the disease described here. The pathogenic processes in dermatomyositis are humorally mediated and centered primarily on vessels, with little evidence of lymphocytes invading nonnecrotic muscle fibers. Microangiopathy leading to a net reduction in number of capillaries per muscle fiber is an early sign and precedes the inflammatory process and muscle damage (37). Ischemic involvement of larger intramuscular blood vessels is probably responsible for perifascicular atrophy. We could not detect any overt perivascular inflammation or perifascicular atrophy in the muscles in this model, confirming that it more closely resembles polymyositis.
Epidemiological study of the events preceding the onset of clinical myositis (43) and attempts to find a persistent inciting virus (3) have failed to point to a specific pathway or a specific inciting agent for the disease. The results here suggest that no single starting point would be anticipated, even in a group of patients sharing a common clinical phenotype and disease-specific autoantibody such as anti-Jo-1 (44).
In experimental systems, a number of different events as diverse as denervation, cytokine or chemokine stimulation, and viral infection can lead to transient changes in the expression of MHC on the surface of muscle cells. Because antecedent infection or muscle injury are not usually followed by sustained myositis, one may speculate that only prolonged up-regulation of MHC class I, perhaps only in individuals with certain genetic backgrounds, is needed to provoke self-sustained inflammation.
The disease in H+ T+ mice could not be reversed by readministering doxycycline after the onset of clinical disease, probably because of the activation of transgene-independent genes such as endogenous H-2Kb, ICAM-1, and the proinflammatory MIP-1α, MCP-1, and IL-15. It is likely that the process is sustained by the presence of MIP-1α, which can up-regulate MHC class I on human skeletal muscle cells (18), and of MCP-1 (45) and IL-15, which can attract and activate macrophages and T cells (46).
Lymphocytic infiltration is thought to be important in the pathogenesis of the human disease. In support of this view are the very common finding of lymphocytes in biopsies (47), the close approximation of lymphocytes to muscle cells in myositis (48), and the response of the clinical illness to glucocorticoids and other anti-inflammatory agents. On the other hand, lymphocytes are absent in some biopsies; some of the changes in muscle cells, such as the up-regulation of MHC class I, occur away from lymphocytic infiltration (17); and the response to inflammatory drugs, even large doses of glucocorticoids, is often unsatisfactory. This transgenic mouse model seems to resemble the latter group of patients.
This model suggests that the presumed order of events as an autoimmune disease develops needs to be reconsidered. In particular, an autoimmune disease may unfold in a highly specific pattern as the consequence of an apparently nonspecific event. The sustained up-regulation of MHC class I in a tissue, and the specificity of the autoantibodies may derive not from the specificity of the stimulus, but from its context, location, and probably its duration.
Acknowledgments
We thank Craig Hyde of the Laboratory of Physical Biology for purifying recombinant human HRS, and John O'Shea of the Arthritis and Rheumatism Branch for useful discussions. Both are affiliated with the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health. We also thank members of the Transgenic Mouse Facility, National Institutes of Mental Health, National Institutes of Health for generating the H+ mice.
Abbreviations
- HRS
histidyl-tRNA synthetase
- TRE
tetracycline-responsive element
- tTA
tetracycline transactivator
- MCK
muscle-specific creatine kinase
- ICAM-1
intercellular adhesion molecule-1
- MIP
marcophage inflammatory protein
- MCP
monocyte chemotactic protein
- TGF
transforming growth factor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 8750.
References
- 1.Targoff I N. Curr Opin Rheumatol. 1989;1:432–442. doi: 10.1097/00002281-198901040-00004. [DOI] [PubMed] [Google Scholar]
- 2.Gershwin M E, Mackay I R. Gastroenterology. 1991;100:822–833. doi: 10.1016/0016-5085(91)80033-6. [DOI] [PubMed] [Google Scholar]
- 3.Leff R L, Love L A, Miller F W, Greenberg S J, Klein E A, Dalakas M C, Plotz P H. Lancet. 1992;339:1192–1195. doi: 10.1016/0140-6736(92)91134-t. [DOI] [PubMed] [Google Scholar]
- 4.Miller F W, Twitty S A, Biswas T, Plotz P H. J Clin Invest. 1990;85:468–475. doi: 10.1172/JCI114461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foulis A K, Farquharson M A, Hardman R. Diabetologia. 1987;30:333–343. doi: 10.1007/BF00299027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanafusa T, Pujol-Borrell R, Chiovato L, Russell R C, Doniach D, Bottazzo G F. Lancet. 1983;2:1111–1115. doi: 10.1016/s0140-6736(83)90628-1. [DOI] [PubMed] [Google Scholar]
- 7.Bottazzo G F, Dean B M, McNally J M, MacKay E H, Swift P G, Gamble D R. N Engl J Med. 1985;313:353–360. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- 8.Allison J, Campbell I L, Morahan G, Mandel T E, Harrison L C, Miller J F. Nature (London) 1988;333:529–533. doi: 10.1038/333529a0. [DOI] [PubMed] [Google Scholar]
- 9.Turnley A M, Morahan G, Okano H, Bernard O, Mikoshiba K, Allison J, Bartlett P F, Miller J F. Nature (London) 1991;353:566–569. doi: 10.1038/353566a0. [DOI] [PubMed] [Google Scholar]
- 10.Frauman A G, Chu P, Harrison L C. Mol Cell Biol. 1993;13:1554–1564. doi: 10.1128/mcb.13.3.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kouskoff V, Korganow A S, Duchatelle V, Degott C, Benoist C, Mathis D. Cell. 1996;87:811–822. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 12.Korganow A S, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, Degott C, Kikutani H, Rajewsky K, Pasquali J L, et al. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto I, Staub A, Benoist C, Mathis D. Science. 1999;286:1732–1735. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]
- 14.Yaswen L, Kulkarni A B, Fredrickson T, Mittleman B, Schiffman R, Payne S, Longenecker G, Mozes E, Karlsson S. Blood. 1996;87:1439–1445. [PubMed] [Google Scholar]
- 15.Cohen P L, Eisenberg R A. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 16.Portanova J P, Ebling F M, Hammond W S, Hahn B H, Kotzin B L. J Immunol. 1988;141:3370–3376. [PubMed] [Google Scholar]
- 17.Emslie-Smith A M, Arahata K, Engel A G. Hum Pathol. 1989;20:224–231. doi: 10.1016/0046-8177(89)90128-7. [DOI] [PubMed] [Google Scholar]
- 18.Nagaraju K, Raben N, Merritt G, Loeffler L, Kirk K, Plotz P. Clin Exp Immunol. 1998;113:407–414. doi: 10.1046/j.1365-2249.1998.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lundberg I, Brengman J M, Engel A G. J Neuroimmunol. 1995;63:9–16. doi: 10.1016/0165-5728(95)00122-0. [DOI] [PubMed] [Google Scholar]
- 20.Adams E M, Kirkley J, Eidelman G, Dohlman J, Plotz P H. Proc Assoc Am Physicians. 1997;109:275–285. [PubMed] [Google Scholar]
- 21.Weiss E H, Mellor A, Golden L, Fahrner K, Simpson E, Hurst J, Flavell R A. Nature (London) 1983;301:671–674. doi: 10.1038/301671a0. [DOI] [PubMed] [Google Scholar]
- 22.Ghersa P, Gobert R P, Sattonnet-Roche P, Richards C A, Merlo Pich E, Hooft van Huijsduijnen R. Gene Ther. 1998;5:1213–1220. doi: 10.1038/sj.gt.3300713. [DOI] [PubMed] [Google Scholar]
- 23.Raben N, Nichols R, Dohlman J, McPhie P, Sridhar V, Hyde C, Leff R, Plotz P. J Biol Chem. 1994;269:24277–24283. [PubMed] [Google Scholar]
- 24.Ait-Azzouzene D, Langkopf A, Cohen J, Bleux C, Gendron M C, Kanellopoulos-Langevin C. Mol Reprod Dev. 1998;50:35–44. doi: 10.1002/(SICI)1098-2795(199805)50:1<35::AID-MRD5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 25.Hohlfeld R, Engel A G. Immunol Today. 1994;15:269–274. doi: 10.1016/0167-5699(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 26.Streilein J W. Curr Opin Immunol. 1993;5:428–432. doi: 10.1016/0952-7915(93)90064-y. [DOI] [PubMed] [Google Scholar]
- 27.Schneider C, Gold R, Dalakas M C, Schmied M, Lassmann H, Toyka K V, Hartung H P. J Neuropathol Exp Neurol. 1996;55:1205–1209. doi: 10.1097/00005072-199612000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Olive M, Martinez-Matos J A, Montero J, Ferrer I. Muscle Nerve. 1997;20:1328–1330. doi: 10.1002/(sici)1097-4598(199710)20:10<1328::aid-mus20>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 29.Inukai A, Kobayashi Y, Ito K, Doyu M, Takano A, Honda H, Sobue G. Muscle Nerve. 1997;20:702–709. doi: 10.1002/(sici)1097-4598(199706)20:6<702::aid-mus7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 30.Behrens L, Bender A, Johnson M A, Hohlfeld R. Brain. 1997;120:929–938. doi: 10.1093/brain/120.6.929. [DOI] [PubMed] [Google Scholar]
- 31.Tews D S, Goebel H H. Clin Immunol Immunopathol. 1998;87:240–247. doi: 10.1006/clin.1998.4527. [DOI] [PubMed] [Google Scholar]
- 32.Tschopp J, Irmler M, Thome M. Curr Opin Immunol. 1998;10:552–558. doi: 10.1016/s0952-7915(98)80223-9. [DOI] [PubMed] [Google Scholar]
- 33.Goebels N, Michaelis D, Wekerle H, Hohlfeld R. J Immunol. 1992;149:661–667. [PubMed] [Google Scholar]
- 34.Fu T M, Ulmer J B, Caulfield M J, Deck R R, Friedman A, Wang S, Liu X, Donnelly J J, Liu M A. Mol Med. 1997;3:362–371. [PMC free article] [PubMed] [Google Scholar]
- 35.Wakasugi K, Schimmel P. Science. 1999;284:147–151. doi: 10.1126/science.284.5411.147. [DOI] [PubMed] [Google Scholar]
- 36.Naparstek Y, Plotz P H. Annu Rev Immunol. 1993;11:79–104. doi: 10.1146/annurev.iy.11.040193.000455. [DOI] [PubMed] [Google Scholar]
- 37.Emslie-Smith A M, Engel A G. Ann Neurol. 1990;27:343–356. doi: 10.1002/ana.410270402. [DOI] [PubMed] [Google Scholar]
- 38.De Visser M, Emslie-Smith A M, Engel A G. J Neurol Sci. 1989;94:181–192. doi: 10.1016/0022-510x(89)90228-1. [DOI] [PubMed] [Google Scholar]
- 39.Estruch R, Grau J M, Fernandez-Sola J, Casademont J, Monforte R, Urbano-Marquez A. Hum Pathol. 1992;23:888–895. doi: 10.1016/0046-8177(92)90400-w. [DOI] [PubMed] [Google Scholar]
- 40.Karpati G, Pouliot Y, Carpenter S. Ann Neurol. 1988;23:64–72. doi: 10.1002/ana.410230111. [DOI] [PubMed] [Google Scholar]
- 41.Arahata K, Engel A G. Ann Neurol. 1984;16:193–208. doi: 10.1002/ana.410160206. [DOI] [PubMed] [Google Scholar]
- 42.Goebels N, Michaelis D, Engelhardt M, Huber S, Bender A, Pongratz D, Johnson M A, Wekerle H, Tschopp J, Jenne D, et al. J Clin Invest. 1996;97:2905–2910. doi: 10.1172/JCI118749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyon M G, Bloch D A, Hollak B, Fries J F. J Rheumatol. 1989;16:1218–1224. [PubMed] [Google Scholar]
- 44.Love L A, Leff R L, Fraser D D, Targoff I N, Dalakas M C, Plotz P H, Miller F W. Medicine (Baltimore) 1991;70:360–374. doi: 10.1097/00005792-199111000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Tesch G H, Maifert S, Schwarting A, Rollins B J, Kelley V R. J Exp Med. 1999;190:1813–1824. doi: 10.1084/jem.190.12.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McInnes I B, Liew F Y. Immunol Today. 1998;19:75–79. doi: 10.1016/s0167-5699(97)01205-x. [DOI] [PubMed] [Google Scholar]
- 47.Engel A G, Arahata K. Hum Pathol. 1986;17:704–721. doi: 10.1016/s0046-8177(86)80180-0. [DOI] [PubMed] [Google Scholar]
- 48.Goebels N, Michaelis D, Engelhardt M, Huber S, Bender A, Pongratz D, Johnson M, Wekerle H, Tschopp J, Jenne D, et al. J Clin Invest. 1996;97:2905–2910. doi: 10.1172/JCI118749. [DOI] [PMC free article] [PubMed] [Google Scholar]