Abstract
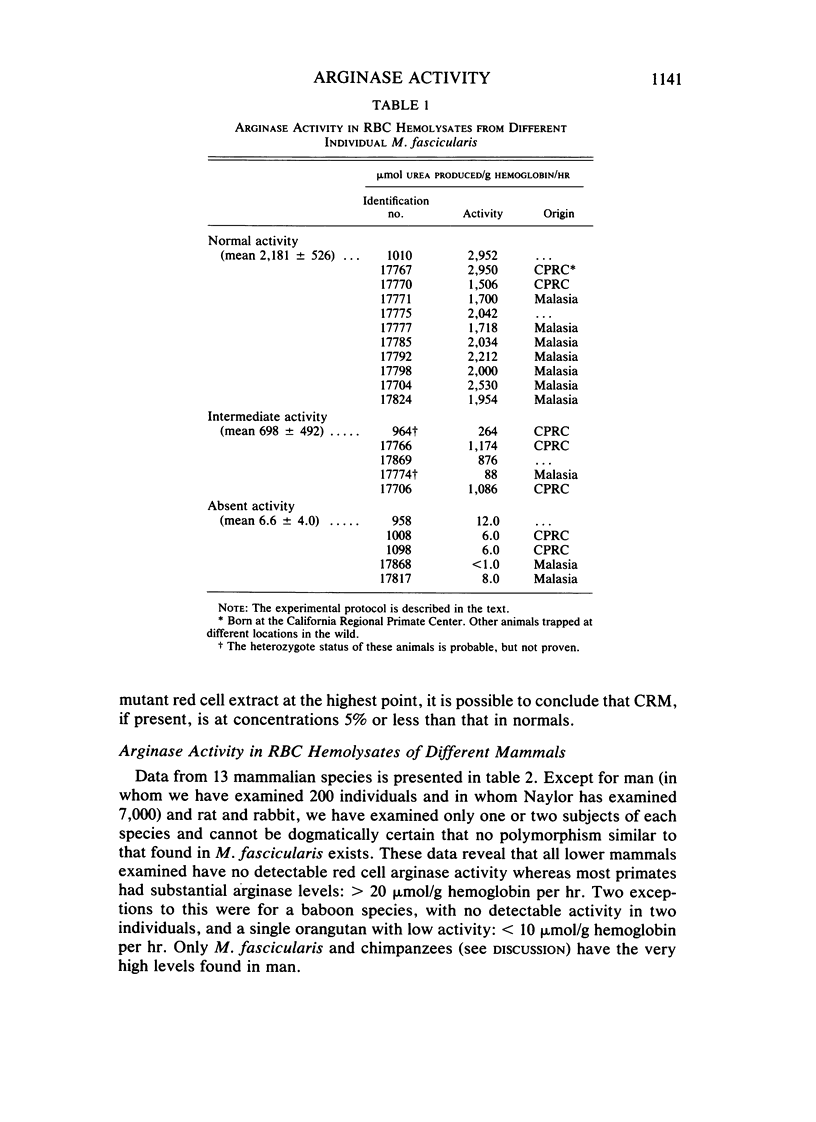
Arginase activity in red blood cells (RBC) of various mammalian species including man was determined. In nonprimate species, the activity generally fell below the level of detectability of the assay: less than 1.0 mumol urea/g hemoglobin per hr. Activities in higher nonhuman primates were equal to or of the same order of magnitude as those in man (approximately 950 mumol/g hemoglobin per hr). RBC arginase deficiency with normal liver arginase activity has been shown to segregate as an autosomal codominant trait in Macaca fascicularis established and bred in captivity. This study confirms the presence of this polymorphism in wild populations trapped in several geographic areas and demonstrates the absence of immunologically cross-reactive material in the RBC of RBC arginase-deficient animals. These data when taken together suggest that the expression of arginase in RBC is the result of a regulatory alteration, has evolved under positive selective pressure, and is not an example of the vestigial persistence of an arcane function. The expression of arginase in the RBC results in a marked drop in the arginine content of these cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barej W., Hill H. Arginine metabolism in the blood cells of cattle and goats. Q J Exp Physiol Cogn Med Sci. 1970 Jan;55(1):64–68. doi: 10.1113/expphysiol.1970.sp002052. [DOI] [PubMed] [Google Scholar]
- Cederbaum S. D., Shaw K. N., Spector E. B., Verity M. A., Snodgrass P. J., Sugarman G. I. Hyperargininemia with arginase deficiency. Pediatr Res. 1979 Jul;13(7):827–833. doi: 10.1203/00006450-197907000-00007. [DOI] [PubMed] [Google Scholar]
- Michels V. V., Beaudet A. L. Arginase deficiency in multiple tissues in argininemia. Clin Genet. 1978 Jan;13(1):61–67. doi: 10.1111/j.1399-0004.1978.tb04128.x. [DOI] [PubMed] [Google Scholar]
- Shih V. E., Jones T. C., Levy H. L., Madigan P. M. Arginase deficiency in Macaca fascicularis. I. Arginase activity and arginine concentration in erythrocytes and liver. Pediatr Res. 1972 Jun;6(6):548–551. doi: 10.1203/00006450-197206000-00003. [DOI] [PubMed] [Google Scholar]
- Spector E. B., Kiernan M., Bernard B., Cederbaum S. D. Properties of fetal and adult red blood cell arginase: a possible prenatal diagnostic test for arginase deficiency. Am J Hum Genet. 1980 Jan;32(1):79–87. [PMC free article] [PubMed] [Google Scholar]
- Spector E. B., Rice S. C., Cederbaum S. D. Immunologic studies of arginase in tissues of normal human adult and arginase-deficient patients. Pediatr Res. 1983 Dec;17(12):941–944. doi: 10.1203/00006450-198312000-00003. [DOI] [PubMed] [Google Scholar]
- Terasaki K., Spector E. B., Hendrickson R., Cederbaum S. D. Properties of arginase from liver of Macaca fascicularis; comparison of normals with red blood cell arginase deficient monkeys. Biochem Genet. 1980 Oct;18(9-10):829–841. doi: 10.1007/BF00500116. [DOI] [PubMed] [Google Scholar]


