Abstract
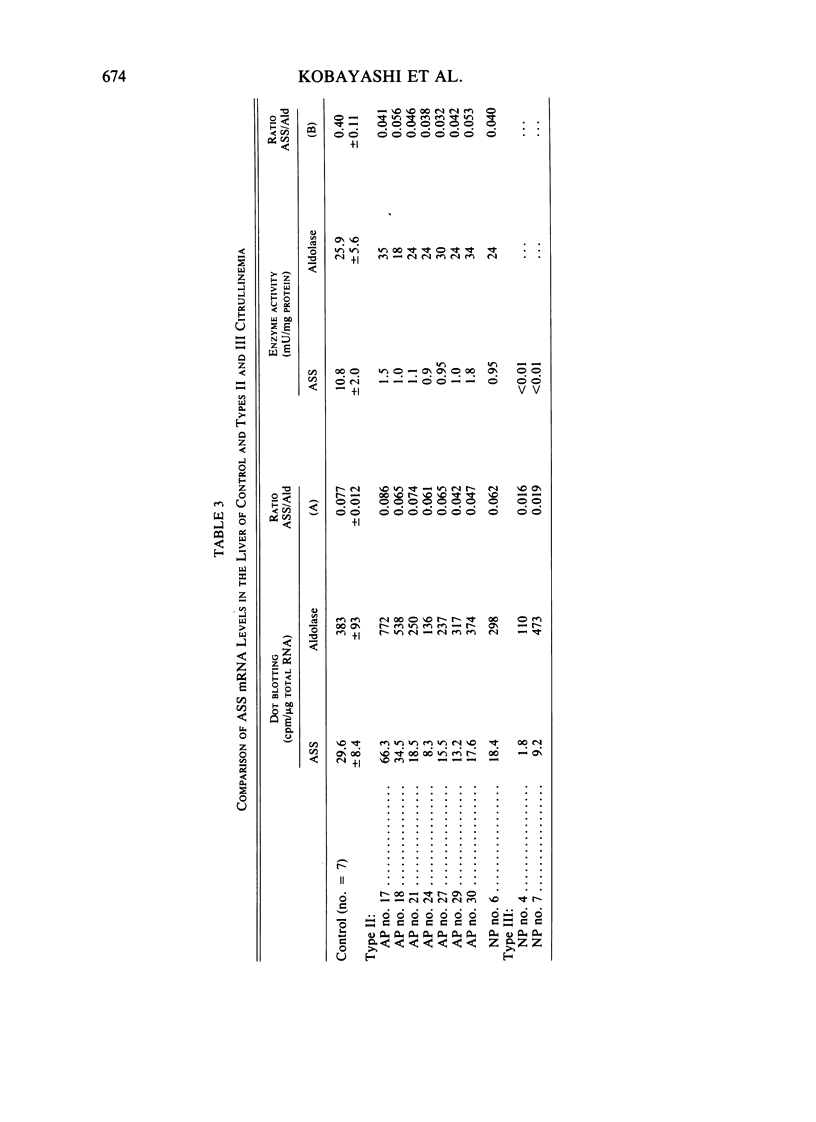
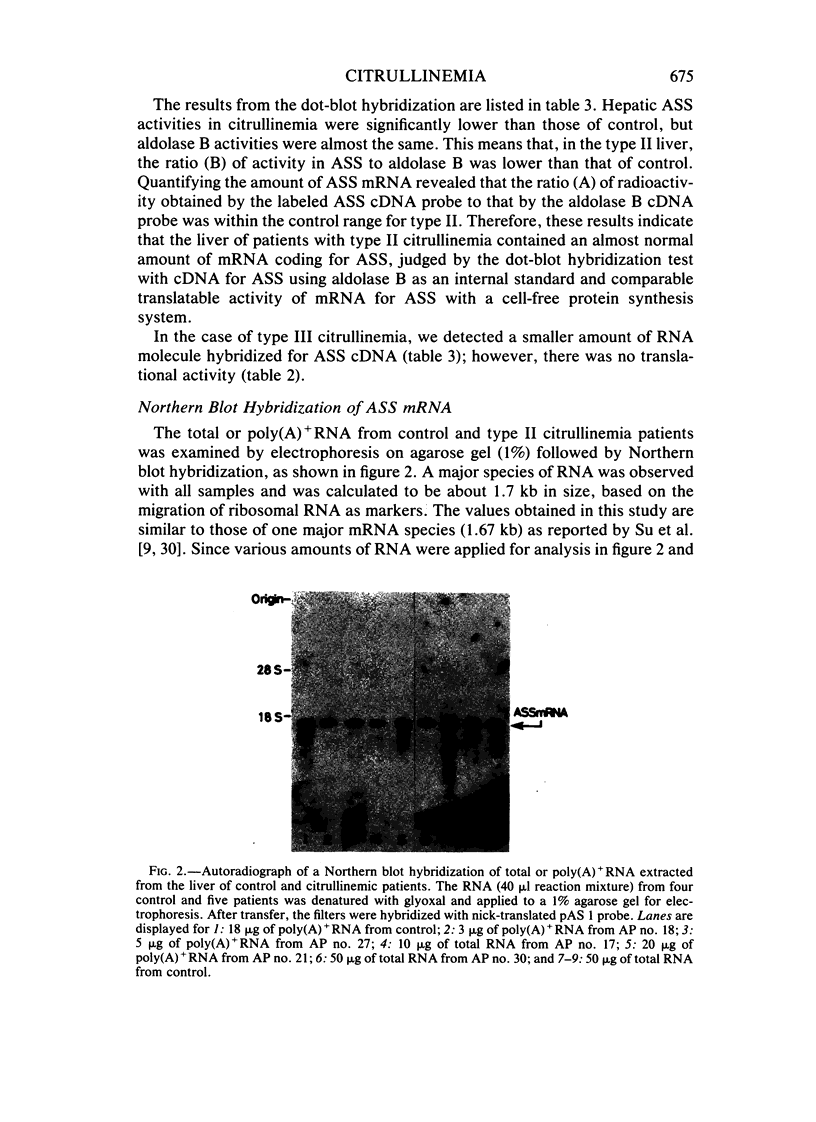
Messenger RNA coding for argininosuccinate synthetase (ASS), extracted from the livers of some patients with citrullinemia, was analyzed using a cell-free translation system and dot and Northern blot hybridization with cDNA probe for ASS. In patients with quantitative-type citrullinemia, called type II here, previous studies have demonstrated that the hepatic content of the enzyme was about 10% of the control value, whereas the translatable mRNA level for the enzyme was similar to that of control livers. Here, we confirmed that the type II liver contained an almost normal amount of mRNA coding for ASS, judged by the dot-blot hybridization technique with cDNA. Northern blot hybridization of RNA indicated that there was hybridizable mRNA of approximately normal size (about 1.7 kilobase [kb]) in each, suggesting that large structural gene deletions had not occurred. These results indicate that in type II citrullinemia, the decrease in the enzyme protein is due either to increased degradation of the enzyme or to decreased or inhibited translation in the liver. Another type of citrullinemia was found and classified as type III. It is characterized by no detectable enzyme activity for ASS or translation activity for ASS mRNA. However, a smaller amount of RNA molecule hybridized for ASS cDNA was detected.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaboshi I., Endo F., Matsuda I., Saheki T. Kinetic analysis of argininosuccinate synthetase in a variant form of citrullinaemia. J Inherit Metab Dis. 1983;6(1):36–39. doi: 10.1007/BF02391191. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock H. G., Su T. S., O'Brien W. E., Beaudet A. L. Sequence for human argininosuccinate synthetase cDNA. Nucleic Acids Res. 1983 Sep 24;11(18):6505–6512. doi: 10.1093/nar/11.18.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand P., Miura S., Mori M., Cathelineau L., Kamoun P., Tatibana M. Cell-free synthesis and transport of precursors of mutant ornithine carbamoyltransferases into mitochondria. Biochim Biophys Acta. 1983 Nov 8;760(3):389–397. doi: 10.1016/0304-4165(83)90379-3. [DOI] [PubMed] [Google Scholar]
- Carritt B., Goldfarb P. S., Hooper M. L., Slack C. Chromosome assignment of a human gene for argininosuccinate synthetase expression in Chinese hamsterxhuman somatic cell hybrids. Exp Cell Res. 1977 Apr;106(1):71–78. doi: 10.1016/0014-4827(77)90242-7. [DOI] [PubMed] [Google Scholar]
- Carritt B., Povey S. Regional asssignments of the loci AK3, ACONS, and ASS on human chromosome 9. Cytogenet Cell Genet. 1979;23(3):171–181. doi: 10.1159/000131323. [DOI] [PubMed] [Google Scholar]
- Carritt B. Somatic cell genetic evidence for the presence of a gene for citrullinemia on human chromosome 9. Cytogenet Cell Genet. 1977;19(1):44–48. doi: 10.1159/000130793. [DOI] [PubMed] [Google Scholar]
- Crkvenjakov R., Maksimović V., Glisin V. A pool of nonpolysomal globin mRNAs in globin deficient reticulocytes of the anemic Belgrade rat. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1524–1531. doi: 10.1016/0006-291x(82)90961-5. [DOI] [PubMed] [Google Scholar]
- Esumi H., Takahashi Y., Sato S., Nagase S., Sugimura T. A seven-base-pair deletion in an intron of the albumin gene of analbuminemic rats. Proc Natl Acad Sci U S A. 1983 Jan;80(1):95–99. doi: 10.1073/pnas.80.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freytag S. O., Beaudet A. L., Bock H. G., O'Brien W. E. Molecular structure of the human argininosuccinate synthetase gene: occurrence of alternative mRNA splicing. Mol Cell Biol. 1984 Oct;4(10):1978–1984. doi: 10.1128/mcb.4.10.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freytag S. O., Bock H. G., Beaudet A. L., O'Brien W. E. Molecular structures of human argininosuccinate synthetase pseudogenes. Evolutionary and mechanistic implications. J Biol Chem. 1984 Mar 10;259(5):3160–3166. [PubMed] [Google Scholar]
- Glynias M. J., Morris S. M., Jr, Fantozzi D. A., Winberry L. K., Back D. W., Fisch J. E., Goodridge A. G. A cloned cDNA for duck malic enzyme detects abnormally large malic enzyme mRNAs in a strain of mice (Mod-1n) that does not express malic enzyme protein. Biochemistry. 1984 Jul 17;23(15):3454–3459. doi: 10.1021/bi00310a011. [DOI] [PubMed] [Google Scholar]
- Gracy R. W., Lacko A. G., Brox L. W., Adelman R. C., Horecker B. L. Structural relations in aldolases purified from rat liver and muscle and Novikoff hepatoma. Arch Biochem Biophys. 1970 Feb;136(2):480–490. doi: 10.1016/0003-9861(70)90219-5. [DOI] [PubMed] [Google Scholar]
- Harding J. D., Przybyla A. E., MacDonald R. J., Pictet R. L., Rutter W. J. Effects of dexamethasone and 5-bromodeoxyuridine on the synthesis of amylase mRNA during pancreatic development in vitro. J Biol Chem. 1978 Oct 25;253(20):7531–7537. [PubMed] [Google Scholar]
- Jinno Y., Nomiyama H., Wakasugi S., Shimada K., Matsuda I., Saheki T. Isolation and characterization of phage clones carrying the human argininosuccinate synthetase-like genes. J Inherit Metab Dis. 1984;7(3):133–134. doi: 10.1007/BF01801773. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
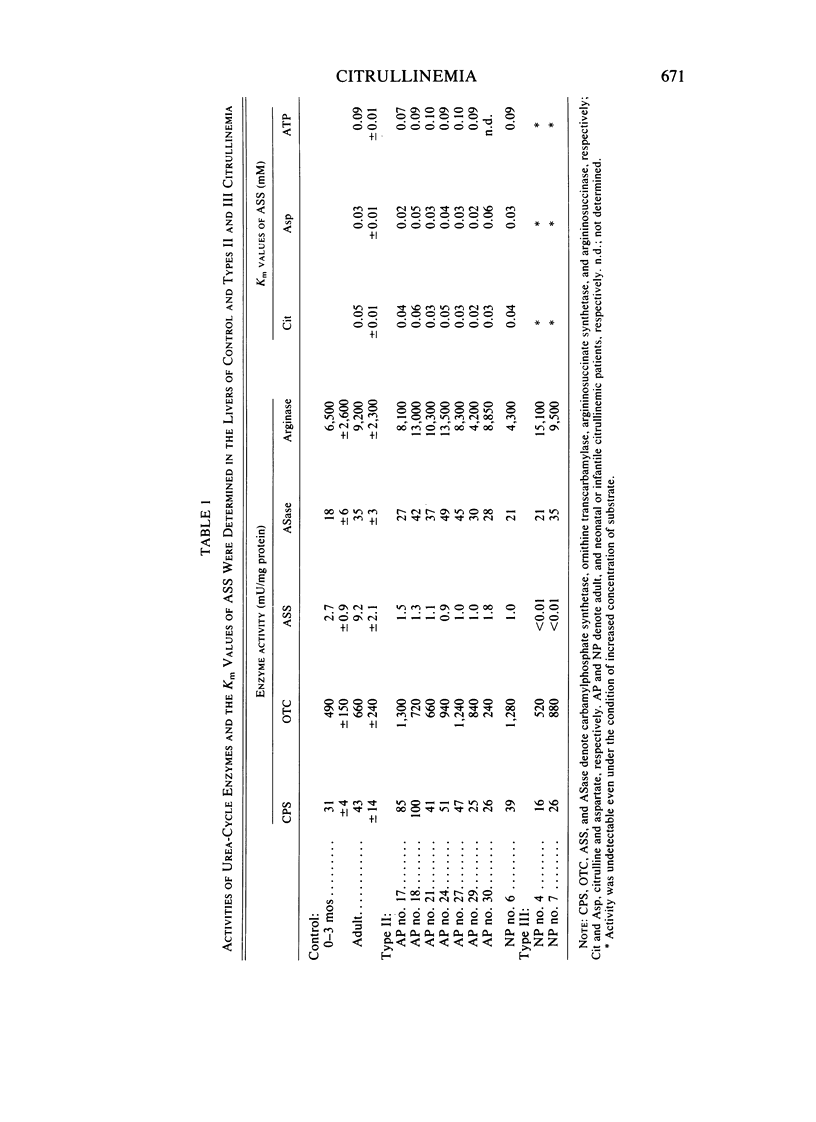
- Maniatis T., Fritsch E. F., Lauer J., Lawn R. M. The molecular genetics of human hemoglobins. Annu Rev Genet. 1980;14:145–178. doi: 10.1146/annurev.ge.14.120180.001045. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA). Proc Natl Acad Sci U S A. 1984 Apr;81(7):1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Miura S., Tatibana M., Cohen P. P. Cell-free translation of carbamyl phosphate synthetase I and ornithine transcarbamylase messenger RNAs of rat liver. Effect of dietary protein and fasting on translatable mRNA levels. J Biol Chem. 1981 Apr 25;256(8):4127–4132. [PubMed] [Google Scholar]
- Mori M., Morris S. M., Jr, Cohen P. P. Cell-free translation and thyroxine induction of carbamyl phosphate synthetase I messenger RNA in tadpole liver. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3179–3183. doi: 10.1073/pnas.76.7.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueckler M. M., Merrill M. J., Pitot H. C. Translational and pretranslational control of ornithine aminotransferase synthesis in rat liver. J Biol Chem. 1983 May 25;258(10):6109–6114. [PubMed] [Google Scholar]
- Mueckler M. M., Pitot H. C. Structure and function of rat liver polysome populations. I. Complexity, frequency distribution, and degree of uniqueness of free and membrane-bound polysomal polyadenylate-containing RNA populations. J Cell Biol. 1981 Aug;90(2):495–506. doi: 10.1083/jcb.90.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pestka S., Daugherty B. L., Jung V., Hotta K., Pestka R. K. Anti-mRNA: specific inhibition of translation of single mRNA molecules. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7525–7528. doi: 10.1073/pnas.81.23.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. E., Kalousek F., Orsulak M. D. Biogenesis of ornithine transcarbamylase in spfash mutant mice: two cytoplasmic precursors, one mitochondrial enzyme. Science. 1983 Oct 28;222(4622):426–428. doi: 10.1126/science.6623083. [DOI] [PubMed] [Google Scholar]
- Saheki T., Imamura Y., Inoue I., Miura S., Mori M., Ohtake A., Tatibana M., Katsumata N., Ohno T. Molecular basis of ornithine transcarbamylase deficiency lacking enzyme protein. J Inherit Metab Dis. 1984;7(1):2–8. doi: 10.1007/BF01805609. [DOI] [PubMed] [Google Scholar]
- Saheki T., Nakano K., Kobayashi K., Imamura Y., Itakura Y., Sase M., Hagihara S., Matuo S. Analysis of the enzyme abnormality in eight cases of neonatal and infantile citrullinaemia in Japan. J Inherit Metab Dis. 1985;8(3):155–156. doi: 10.1007/BF01819306. [DOI] [PubMed] [Google Scholar]
- Saheki T., Sase M., Nakano K., Azuma F., Katsunuma T. Some properties of argininosuccinate synthetase purified from human liver and a comparison with the rat liver enzyme. J Biochem. 1983 Jun;93(6):1531–1537. doi: 10.1093/oxfordjournals.jbchem.a134291. [DOI] [PubMed] [Google Scholar]
- Saheki T., Ueda A., Hosoya M., Kusumi K., Takada S., Tsuda M., Katsunuma T. Qualitative and quantitative abnormalities of argininosuccinate synthetase in citrullinemia. Clin Chim Acta. 1981 Feb 5;109(3):325–335. doi: 10.1016/0009-8981(81)90318-1. [DOI] [PubMed] [Google Scholar]
- Saheki T., Ueda A., Iizima K., Yamada N., Kobayashi K., Takahashi K., Katsunuma T. Argininosuccinate synthetase activity in cultured skin fibroblasts of citrullinemic patients. Clin Chim Acta. 1982 Jan 5;118(1):93–97. doi: 10.1016/0009-8981(82)90230-3. [DOI] [PubMed] [Google Scholar]
- Sargent T. D., Dawid I. B. Differential gene expression in the gastrula of Xenopus laevis. Science. 1983 Oct 14;222(4620):135–139. doi: 10.1126/science.6688681. [DOI] [PubMed] [Google Scholar]
- Sase M., Kobayashi K., Imamura Y., Saheki T., Nakano K., Miura S., Mori M. Level of translatable messenger RNA coding for argininosuccinate synthetase in the liver of the patients with quantitative-type citrullinemia. Hum Genet. 1985;69(2):130–134. doi: 10.1007/BF00293282. [DOI] [PubMed] [Google Scholar]
- Simons R. W., Kleckner N. Translational control of IS10 transposition. Cell. 1983 Sep;34(2):683–691. doi: 10.1016/0092-8674(83)90401-4. [DOI] [PubMed] [Google Scholar]
- Su T. S., Beaudet A. L., O'Brien W. E. Abnormal mRNA for argininosuccinate synthetase in citrullinaemia. Nature. 1983 Feb 10;301(5900):533–534. doi: 10.1038/301533a0. [DOI] [PubMed] [Google Scholar]
- Su T. S., Bock H. G., Beaudet A. L., O'Brien W. E. Molecular analysis of argininosuccinate synthetase deficiency in human fibroblasts. J Clin Invest. 1982 Dec;70(6):1334–1339. doi: 10.1172/JCI110736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T. S., Bock H. G., O'Brien W. E., Beaudet A. L. Cloning of cDNA for argininosuccinate synthetase mRNA and study of enzyme overproduction in a human cell line. J Biol Chem. 1981 Nov 25;256(22):11826–11831. [PubMed] [Google Scholar]
- Sul H. S., Wise L. S., Brown M. L., Rubin C. S. Cloning of cDNA sequences for murine malic enzyme and the identification of aberrantly large malic enzyme mRNA in MOD-1 null mice. J Biol Chem. 1984 Jan 10;259(1):555–559. [PubMed] [Google Scholar]
- Takada S., Kusumi T., Saheki T., Tsuda M., Katsunuma T. Studies of rat liver argininosuccinate synthetase. The presence of three forms, and their physicochemical, catalytic, and immunochemical properties. J Biochem. 1979 Nov;86(5):1353–1359. doi: 10.1093/oxfordjournals.jbchem.a132652. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Wiginton D. A., Adrian G. S., Friedman R. L., Suttle D. P., Hutton J. J. Cloning of cDNA sequences of human adenosine deaminase. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7481–7485. doi: 10.1073/pnas.80.24.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenofsky R., Bergmann I., Brawerman G. Cloned complementary deoxyribonucleic acid probes for untranslated messenger ribonucleic acid components of mouse sarcoma ascites cells. Biochemistry. 1982 Aug 17;21(17):3909–3913. doi: 10.1021/bi00260a001. [DOI] [PubMed] [Google Scholar]