Abstract
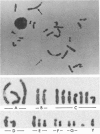
We have developed an improved method for analyzing human sperm chromosome, using zona-free hamster ova. Our main improvements of methodology are as follows: (1) Fertilization rate of hamster oocytes by human spermatozoa was markedly raised by successive treatments of the spermatozoa with 5-15 microM ionophore A23187 solutions and a capacitation medium (BWW medium) containing 3.5% HSA. The HSA most effective in inducing capacitation was selected from several kinds of HSA products commercially available. (2) Monospermic fertilization was ensured by inseminating oocytes with highly capacitated spermatozoa at a low concentration for a short time. (3) TC medium 199 was used for postinsemination culture of the eggs. (4) A medium containing podophyllotoxin and vinblastine (0.04 micrograms/ml each) was used to block karyogamy and first-cleavage spindle formation. (5) Chromosome slides were prepared with our gradual fixation-air-dry method instead of Tarkowski's method. Ninety-two to 177 spermatozoa corresponding in number to 43%-79% (mean: 62%) of the inseminated oocytes were successfully karyotyped in each experiment. In spite of above-mentioned quantitative improvements, quality of Q-banding was not necessarily satisfactory in our slides. Improvement of banding technique is an important problem to be solved in our method. Spontaneous incidence of chromosome aberrations was studied in a total of 1,091 spermatozoa obtained from nine semen samples from four donors. Incidences of aneuploidy and structural anomaly were 0.9% (hyperhaploidy, 0.45%; hypohaploidy, 0.45%) and 13.0%, respectively. Structural aberrations included breaks (45.1%), fragments (32.4%), exchanges (21.8%), and deletions (0.7%). Ratio of X-sperm to Y-sperm was 53% to 47%. These results were discussed in comparison with those reported previously.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balkan W., Burns K., Martin R. H. Sperm chromosome analysis of a man heterozygous for a pericentric inversion of chromosome 3. Cytogenet Cell Genet. 1983;35(4):295–297. doi: 10.1159/000131882. [DOI] [PubMed] [Google Scholar]
- Balkan W., Martin R. H. Chromosome segregation into the spermatozoa of two men heterozygous for different reciprocal translocations. Hum Genet. 1983;63(4):345–348. doi: 10.1007/BF00274760. [DOI] [PubMed] [Google Scholar]
- Balkan W., Martin R. H. Segregation of chromosomes into the spermatozoa of a man heterozygous for a 14;21 Robertsonian translocation. Am J Med Genet. 1983 Oct;16(2):169–172. doi: 10.1002/ajmg.1320160206. [DOI] [PubMed] [Google Scholar]
- Brandriff B., Gordon L., Ashworth L., Watchmaker G., Carrano A., Wyrobek A. Chromosomal abnormalities in human sperm: comparisons among four healthy men. Hum Genet. 1984;66(2-3):193–201. doi: 10.1007/BF00286600. [DOI] [PubMed] [Google Scholar]
- Brandriff B., Gordon L., Ashworth L., Watchmaker G., Moore D., 2nd, Wyrobek A. J., Carrano A. V. Chromosomes of human sperm: variability among normal individuals. Hum Genet. 1985;70(1):18–24. doi: 10.1007/BF00389451. [DOI] [PubMed] [Google Scholar]
- Edmonds D. K., Lindsay K. S., Miller J. F., Williamson E., Wood P. J. Early embryonic mortality in women. Fertil Steril. 1982 Oct;38(4):447–453. [PubMed] [Google Scholar]
- Fraser L. R., Maudlin I. Analysis of aneuploidy in first-cleavage mouse embryos fertilized in vitro and in vivo. Environ Health Perspect. 1979 Aug;31:141–149. doi: 10.1289/ehp.7931141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. P. The induction of the acrosome reaction in guinea-pig sperm by the divalent metal cation ionophore A23187. J Cell Sci. 1978 Aug;32:137–151. doi: 10.1242/jcs.32.1.137. [DOI] [PubMed] [Google Scholar]
- Hirao Y., Yanagimachi R. Detrimental effect of visible light on meiosis of mammalian eggs in vitro. J Exp Zool. 1978 Dec;206(3):365–369. doi: 10.1002/jez.1402060308. [DOI] [PubMed] [Google Scholar]
- Martin R. H. A detailed method for obtaining preparations of human sperm chromosomes. Cytogenet Cell Genet. 1983;35(4):252–256. doi: 10.1159/000131876. [DOI] [PubMed] [Google Scholar]
- Martin R. H. Analysis of human sperm chromosome complements from a male heterozygous for a reciprocal translocation t(11;22)(q23;q11). Clin Genet. 1984 Apr;25(4):357–361. doi: 10.1111/j.1399-0004.1984.tb02004.x. [DOI] [PubMed] [Google Scholar]
- Martin R. H., Balkan W., Burns K., Rademaker A. W., Lin C. C., Rudd N. L. The chromosome constitution of 1000 human spermatozoa. Hum Genet. 1983;63(4):305–309. doi: 10.1007/BF00274750. [DOI] [PubMed] [Google Scholar]
- Martin R. H., Lin C. C., Balkan W., Burns K. Direct chromosomal analysis of human spermatozoa: preliminary results from 18 normal men. Am J Hum Genet. 1982 May;34(3):459–468. [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y., Yamada T., Tobari I. Studies on chromosome aberrations in the eggs of mice fertilized in vitro after irradiation. I. Chromosome aberrations induced in sperm after X-irradiation. Mutat Res. 1985 Jan-Feb;148(1-2):113–117. doi: 10.1016/0027-5107(85)90214-3. [DOI] [PubMed] [Google Scholar]
- ORSINI M. W., PANSKY B. The natural resistance of the golden hamster to colchicine. Science. 1952 Jan 25;115(2978):88–89. doi: 10.1126/science.115.2978.88. [DOI] [PubMed] [Google Scholar]
- Overstreet J. W., Yanagimachi R., Katz D. F., Hayashi K., Hanson F. W. Penetration of human spermatozoa into the human zona pellucida and the zona-free hamster egg: a study of fertile donors and infertile patients. Fertil Steril. 1980 May;33(5):534–542. doi: 10.1016/s0015-0282(16)44720-5. [DOI] [PubMed] [Google Scholar]
- Perreault S. D., Rogers B. J. Capacitation pattern of human spermatozoa. Fertil Steril. 1982 Aug;38(2):258–260. [PubMed] [Google Scholar]
- Rudak E., Jacobs P. A., Yanagimachi R. Direct analysis of the chromosome constitution of human spermatozoa. Nature. 1978 Aug 31;274(5674):911–913. doi: 10.1038/274911a0. [DOI] [PubMed] [Google Scholar]
- Russell L., Peterson R., Freund M. Direct evidence for formation of hybrid vesicles by fusion of plasma and outer acrosomal membranes during the acrosome reaction in boar spermatozoa. J Exp Zool. 1979 Apr;208(1):41–56. doi: 10.1002/jez.1402080106. [DOI] [PubMed] [Google Scholar]
- Smith M., Peterson R. N., Russell L. D. Penetration of zona-free hamster eggs by boar sperm treated with the ionophore A23187 and inhibition of penetration by antiplasma membrane antibodies. J Exp Zool. 1983 Jan;225(1):157–160. doi: 10.1002/jez.1402250120. [DOI] [PubMed] [Google Scholar]
- Tohda H., Horaguchi K., Takahashi K., Oikawa A., Matsushima T. Epstein-Barr virus-transformed human lymphoblastoid cells for study of sister chromatid exchange and their evaluation as a test system. Cancer Res. 1980 Dec;40(12):4775–4780. [PubMed] [Google Scholar]
- Yanagimachi R., Yanagimachi H., Rogers B. J. The use of zona-free animal ova as a test-system for the assessment of the fertilizing capacity of human spermatozoa. Biol Reprod. 1976 Nov;15(4):471–476. doi: 10.1095/biolreprod15.4.471. [DOI] [PubMed] [Google Scholar]