Abstract
The efficacy and safety of a new immunotherapy protocol for cancer were tested in a severe combined immunodeficient mouse model of human skin and metastatic lung melanoma. The protocol involves intratumoral injections of replication-incompetent adenoviral vectors encoding immunoconjugates composed of the Fc region of an IgG1 immunoglobulin conjugated to a tumor-targeting domain. One targeting domain is factor VII that binds to tissue factor expressed on endothelial cells lining the tumor neovasculature and on tumor cells; the active site of factor VII was mutated to inhibit the initiation of blood coagulation. Another targeting domain is a single-chain Fv antibody that binds to a cognate antigen expressed on human melanoma cells. The adenoviral vectors infect mainly the cells of the injected tumor, which synthesize and secrete the immunoconjugates. The bloodborne immunoconjugates induce a cytolytic immune response against the targeted neovasculature endothelial cells and tumor cells. The mouse model experiments showed that intratumoral delivery of the factor VII immunoconjugate, either alone or together with the single-chain Fv immunoconjugate, resulted in growth inhibition and regression of the injected tumor, and also of distant metastatic tumors, without evidence of damage to normal organs. There was extensive destruction of the tumor neovasculature, presumably mediated by the factor VII immunoconjugate bound to tissue factor on neovasculature endothelial cells. Because tissue factor is generally expressed on neovascular endothelial cells and tumor cells, a factor VII immunoconjugate could be used for immunotherapy against a broad range of human solid tumors.
Adenoviral vectors provide an effective mechanism for establishing and maintaining for several weeks a steady high blood titer of an exogenous protein. The vector can be injected intravenously, resulting in infection mainly of liver cells, or injected directly into a tumor or normal tissue that expresses an adenovirus receptor, resulting in infection mainly of the cells in the injected tissue (1–10). In an earlier study, we showed that i.v. injections of replication-incompetent adenoviral vectors encoding immunoconjugates targeted to tumor vascular endothelial cells and/or tumor cells inhibited growth of skin tumors in a severe combined immunodeficient (SCID) mouse model of human melanoma (11). However, this protocol also caused liver damage, apparently as a result of a continuous high level synthesis of the encoded immunoconjugates by the infected liver cells. Because liver damage could jeopardize the potential application of the protocol for immunotherapy of cancer patients, a safer method of administering an adenoviral vector is needed. In the study reported here, we show that damage to the liver and other organs can be avoided if the vectors encoding the immunoconjugates are injected intratumorally instead of intravenously. The vectors infect mainly cells in the injected tumor, which synthesize and secrete the encoded immunoconjugates into the blood. The secreted immunoconjugates inhibited the growth of the injected skin tumor and also of distant metastatic lung tumors, associated with extensive destruction of the tumor vasculature.
Materials and Methods
Cell Lines.
LXSN, TF, and YUsac2 are human melanoma lines, Caki is a human renal tumor line, LnCap is a human prostate tumor line, A204 is a human neuroblastoma line, BT20 is a human breast tumor line, Colo 357 is a human pancreatic tumor line, MS is a human gastric tumor line, B16F10 is a mouse melanoma line, EMT6 is a mouse mammary tumor line, and 293 is a human kidney line (American Type Culture Collection, catalog no. CRL-1573) used for packaging the adenoviral vectors. The culture medium was DMEM + 10% FBS for all of the tumor lines except LnCap, which was cultured in RPMI 1640 + 10% FBS.
Adenoviral Vectors.
The procedures described in an earlier report (11) for producing and purifying the adenoviral vectors encoding the mfVIIasm and G71-1 immunoconjugates were used in this study with the following modification. After the shuttle vector DNAs were digested with PmeI, instead of an ethanol precipitation step, the DNAs were purified by electrophoresis in agarose gel followed by isolation of the DNA bands using the QIAEX II kit (Qiagen). The vector concentrations in the purified preparations were determined by assays for infectious particles (IP) and infectious units (IU), as follows. (i) The IP assay involves diluting the vector preparation 20-fold in lysis buffer (0.1% SDS in PBS) and measuring absorbance at 260 nm; the conversion to IP is 1 OD unit = 1 × 1012 IP. (ii) The IU assay involves infecting cultures of 293 cells with serial dilutions of the vector preparation, incubating the infected cultures for 2 days, and examining the cells in a fluorescence microscope for expression of the green fluorescent protein (GFP) gene in the vector genome. The IU titer is calculated from the number of cells expressing the GFP gene. The IP and IU titers agreed within ±10%.
Synthesis of the mfVIIasm Immunoconjugate in Tumor Cells.
Tumor cells were grown almost to confluence in 150-mm dishes, and the cells were infected with the adenovirus encoding the mfVIIasm immunoconjugate at a multiplicity of 10 IU per cell. The infected cells were cultured in serum-free medium for 4 days, and 1.5 ml of the medium was mixed with 10 μl of a suspension of protein-A beads (Pierce) and was rotated at 4°C overnight. The bound mfVIIasm immunoconjugate was eluted by heating the beads in 15 μl of SDS/PAGE loading buffer at 80°C for 3 min, and the eluate was fractionated by SDS/PAGE and was transferred to a nitrocellulose membrane. The immunoconjugate band was detected by immunostaining with a goat anti-human or anti-mouse IgG (Fc-specific) probe.
Immunotherapy Tests in Immunodeficient Mice.
Female C.B-17 SCID mice 4–5 weeks old (Taconic Farms) were used for all experiments with immunodeficient mice. Monolayer cultures of the human melanoma lines LXSN or TF were dissociated in PBS + 2 mM EDTA, and were washed and resuspended in PBS. Skin tumors were generated by s.c. injections of 5 × 105 LXSN cells into the right rear flank, and metastatic lung tumors were generated by i.v. injections of 6 × 105 TF cells into the tail vein. The size of the skin tumor was measured in two dimensions with a caliper, and the tumor volume was estimated as (width)2(length)/2. When a skin tumor had grown to a volume of about 100 mm3, intratumoral injections of an adenoviral vector containing a human Fc effector domain was started. For each injection step, a total volume of 50 μl of the vector preparation was injected into three or four sites in the tumor. At the end of the experiment, autopsies were done to collect blood samples and to prepare the tumors and normal organs for morphological and histological examination.
Immunotherapy Tests in Immunocompetent Mice.
Female C57BL/6 mice 4–5 weeks old (Charles River Breeding Laboratories) were used for all experiments involving immunocompetent mice. Monolayer cultures of B16F10 mouse melanoma cells were suspended in PBS + 2 mM EDTA, and were washed and resuspended in PBS. Skin tumors were generated by s.c. injections of 5 × 105 cells into the right rear flank. When the skin tumors had grown to an estimated volume of 140–325 mm3, tail vein injections of the adenoviral vector encoding the mfVIIasm immunoconjugate containing a mouse Fc effector domain were started. The procedures for monitoring the efficacy and safety of the protocol are the same as described above for SCID mice.
Serum Glutamic Oxalacetic Transaminase (SGOT) Assays.
Serum samples collected from mice were frozen and sent to E. Aguilar-Cordova (Baylor College of Medicine, Houston) for analysis of SGOT.
Results
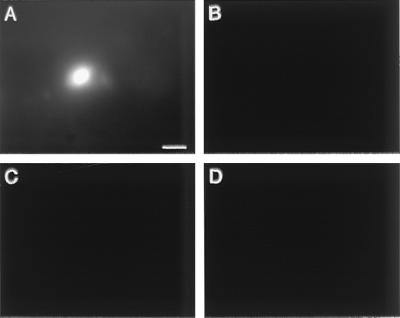
The two immunoconjugates used for this study are composed of a tumor-targeting domain and an effector domain (Fig. 1). The targeting domain is either a mutated mouse factor VII molecule (mfVIIasm) that binds to TF expressed on tumor vascular endothelial cells and tumor cells but does not initiate blood coagulation (11), or the single-chain Fv antibody G71-1 that binds to its cognate antigen expressed selectively on human melanoma cells (12, 13). The effector domain is the Fc region of a human or mouse IgG1 immunoglobulin that induces a cytolytic immune response against the targeted cells (13). The vector for delivering the immunoconjugates is a replication-incompetent adenovirus encoding a secreted form of the immunoconjugates (11). In an earlier study (11), we reported that i.v. injections into SCID mice of the vectors encoding the immunoconjugates inhibited tumor growth but caused histological damage to the liver, which is the primary target for infection by the intravenously injected vector. To determine whether the primary infection could be redirected from liver cells to tumor cells, the control null vector was injected intratumorally into a human skin melanoma growing in SCID mice, and the distribution of the vector in the tumor and liver was mapped by the expression of the green fluorescent protein (GFP) gene inserted into the vector genome (Fig. 2). Intense GFP expression was detected in the tumor but not in the liver, indicating that an injected tumor is the primary target for infection by the vector. The pattern of GFP expression in the tumor appeared to be restricted to a few layers of tumor cells adjacent to the path traversed by the injection needle.
Figure 1.
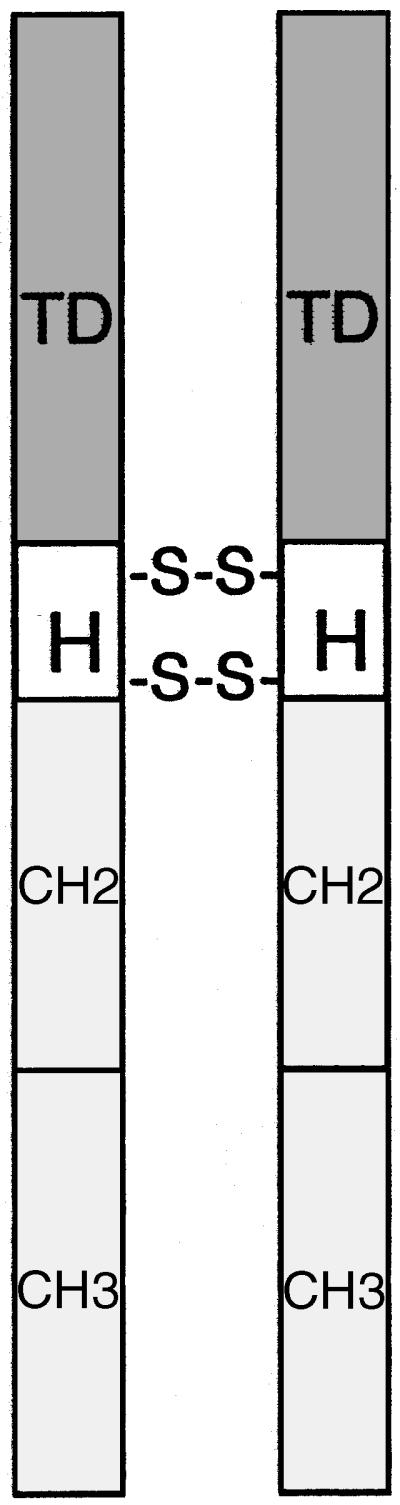
Diagram of an immunoconjugate molecule. TD, targeting domain; H, hinge region of an IgG1 immunoglobulin with 2 disulfide bridges; CH2 and CH3, constant regions of an IgG1 immunoglobulin.
Figure 2.
Distribution of an adenoviral vector in tumor and liver after intratumoral injection. The control vector encoding the GFP protein but not an immunoconjugate was injected into three sites of a human melanoma skin tumor growing in SCID mice. The total vector dose was 6 × 109 IU. The tumor and liver were dissected 40 h after the injection and were examined intact under a dissecting microscope with fluorescence optics. The GFP signal was detected with 480-nm excitation and 630-nm emission, and the background signal was detected with 577-nm excitation and 630-nm emission. (A) Tumor GFP. (B) Tumor background. (C) Liver GFP. (D) Liver background. A bright fluorescent spot similar to the one in A also was detected at two other tumor sites, presumably corresponding to the injection sites. The photographs are focused at one level in the tissues. However, the GFP spot in the tumor also could be detected by focusing above and below that level, suggesting that the tumor cells adjacent to the path traversed by the injection needle are the only cells infected by the vector. (Bar = 50 μm.)
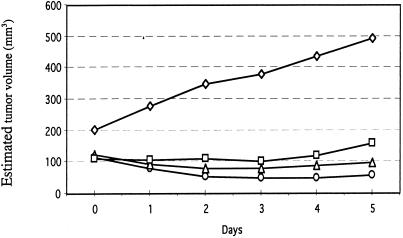
The effect of vector dose on tumor growth was tested by injecting a mixture of the two vectors encoding the mfVIIasm and G71-1 immunoconjugates into a human melanoma skin tumor growing in SCID mice, and measuring the tumor volume during the next 5 days. The ratio of the mfVIIasm vector to the G71-1 vector in the mixture was 5 to 1, to compensate for the higher titer of the G71-1 immunoconjugate secreted into the blood (11). The combined dose of the two vectors used for the injections was varied from 7 × 108 to 6 × 109 infectious units (IU). For a control, an empty vector without an encoded immunoconjugate was injected at a dose of 6 × 109 IU. The strongest inhibition of tumor growth was obtained with the highest dose of the vectors encoding the immunoconjugates (Fig. 3). This vector dose was used for all of the following SCID mouse experiments.
Figure 3.
Dosage effect of intratumorally injected adenoviral vectors on the growth of a human melanoma tumor in SCID mice. The mice were first injected s.c. with the human melanoma cell line LXSN, and when a skin tumor had grown to the size indicated on day 0 the tumor was injected with a mixture of the two adenoviral vectors encoding the mfVIIasm and G71-1 immunoconjugates. The dose for the two vectors was as follows. ○, 6 × 109 IU; ▵, 2 × 109 IU; □, 7 × 108 IU. The dose for the control vector that did not encode an immunoconjugate was as follows. ⋄, 6 × 109 IU.
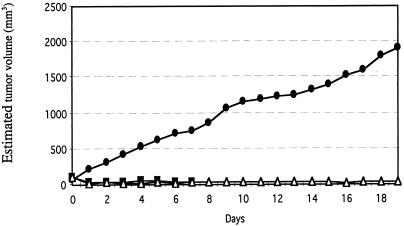
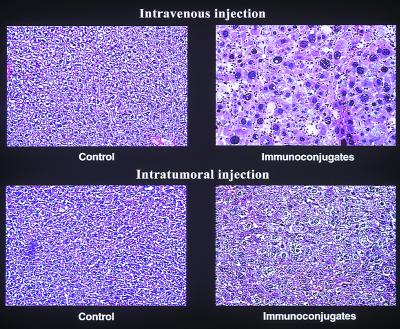
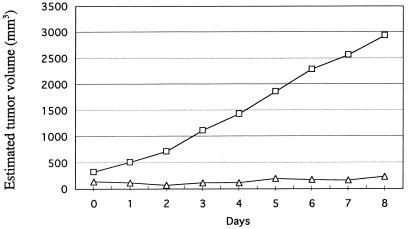
In the next experiment, the inhibitory effect on tumor growth of administering multiple intratumoral injections of the two vectors encoding the mfVIIasm and G71-1 immunoconjugates was monitored for 19 days (Fig. 4). The titer of immunoconjugate proteins in the blood of the mice 2 days after the last injection of the adenoviral vectors ranged from 1 mg/ml to 2 mg/ml, which is about one-fourth the titer produced by intravenously injected vectors (11). The average volume of these tumors decreased by about 70% within 1 day after the first injection of the two vectors, and the volumes did not subsequently increase during the rest of the experiment. Tumors injected with an empty control vector grew continuously, reaching at the end of the experiment an average volume of 1,900 mm3 as compared with 30 mm3 for the tumors injected with the vectors encoding the two immunoconjugates. Tumors injected only with the vector encoding the mfVIIasm immunoconjugate were inhibited almost as strongly as the tumors injected with both vectors, consistent with earlier experiments involving i.v. injections of the vectors (11). The mice remained active throughout the experiment, and in autopsies performed 2 days after the final injections all of the organs appeared morphologically and histologically normal, and there was no evidence of bleeding. Because SCID mice injected intravenously with the vectors encoding the immunoconjugates showed histological damage to the liver cells (11), the livers of the SCID mice injected intratumorally were tested for histological and also functional damage. In contrast to the major histological changes seen in liver sections from the intravenously injected mice, liver sections from the intratumorally injected mice showed relatively minor changes (Fig. 5). Liver function was monitored by assays for the enzyme glutamic oxalacetic transaminase (SGOT) in sera obtained from the mice during autopsy. The levels of SGOT in the control mice and the mice injected with the vectors encoding the immunoconjugates remained within the normal range (210 ± 28 units/liter), indicating that liver function was not impaired.
Figure 4.
Effect of a high dose of intratumorally injected adenoviral vectors on the growth of a human melanoma tumor in SCID mice. The mice were first injected s.c. with the human melanoma line LXSN, and when a skin tumor had grown to the size indicated on day 0 the tumor was injected with 6 × 109 IU of the following adenoviral vectors. ●, control vector (3 mice); ■, vector encoding the mfVIIasm immunoconjugate (6 mice); ▵, mixture of the two vectors encoding the mfVIIasm and G71-1 immunoconjugates (5 mice). Additional injections were done on days 2, 5, 7, 9, 11, 13, 15, and 17. The points on each curve are the average (±20%) of the measurements for all mice in the corresponding group.
Figure 5.
Liver sections from SCID mice injected intravenously or intratumorally with the adenoviral vectors. The i.v. experiment was described previously (see Fig. 5 in ref. 11), and the intratumoral experiment is described in Fig. 4. The panels on the left were injected with the control vector, and the panels on the right were injected with a mixture of the two vectors encoding the mfVIIasm and G71-1 immunoconjugates. The livers were dissected 2 days after the last injection, were fixed in formaldehyde, and were embedded in paraffin. The sections were stained with hematoxylin and eosin and were photographed at a magnification of 100×.
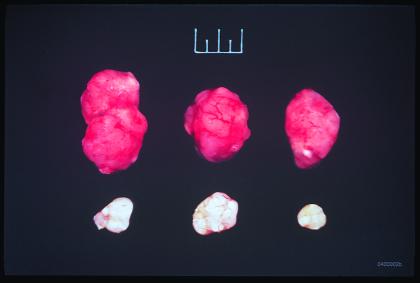
The tumors recovered from the mice injected with the vectors encoding the mfVIIasm and G71-1 immunoconjugates were almost completely devoid of blood vessels (Fig. 6), presumably as the result of a cytolytic immune response against vascular endothelial cells induced by the mfVIIasm immunoconjugate bound to TF.
Figure 6.
Melanoma skin tumors from SCID mice injected intratumorally with the null adenooviral vector control (top row) or with the adenoviral vectors encoding the mfVIIasm and G71-1 immunoconjugates (bottom row). The experiment is described in Fig. 4. The tumors were dissected 2 days after the last injection and were photographed. The full scale at the top of the figure is 1 cm.
The efficacy of the intratumoral injection protocol depends not only on inhibition of the injected skin tumor but also of metastatic tumors that are not accessible for injection. To test for an inhibitory effect on metastatic tumors, we used a SCID mouse model in which human melanoma TF cells are injected into the tail vein, resulting in bloodborne metastases mainly to the lungs. The mice also were injected s.c. with human melanoma LXSN cells at the same time, and the skin tumors that formed 12 days later were injected with the vectors encoding the two immunoconjugates or with an empty control vector. The intratumoral injections were continued on a biweekly schedule for the next 8 weeks, and autopsies were performed 2 days after the last injection to determine the number of tumor nodules on the surface of the lungs (Table 1). The two mice injected with the control vector had 14 and 29 lung nodules, respectively, in contrast to the five mice injected with the vectors encoding the two immunoconjugates, three of which had no lung nodules and two had 1 and 5 nodules, respectively. These results indicate that intratumoral injections of the vectors encoding the two immunoconjugates can inhibit growth of distant metastatic tumors as well as the injected tumor.
Table 1.
Inhibition of metastatic lung tumors by intratumoral injections of adenoviral vectors encoding the immunoconjugates
| Adenoviral vectors | Number of tumors on the surface of the lungs |
|---|---|
| Control | 14, 29 |
| Encoding the immunoconjugates | 1, 5, 0, 0, 0 |
SCID mice were injected on day 0 with human melanoma cells intravenously to generate bloodborne metastatic lung tumors and subcutaneously to generate a skin tumor, as described in the text. Intratumoral injections of the adenoviral vectors encoding the mfVIIasm and G71-1 immunoconjugates into the skin tumor were started on day 12, and additional injections were done biweekly for 8 weeks. The autopsies were done 2 days after the last injection. The number of mice was 2 for the control vector and 5 for the vectors encoding the immunoconjugates.
Another parameter that could affect the efficacy of the protocol in a clinical setting is a patient's immune response to the immunoconjugates or the adenoviral vector. Because the targeting and effector domains of immunoconjugates for clinical use would be derived from human proteins, patients should not mount a significant immune response to the immunoconjugates. However, the adenoviral vector is strongly immunogenic in humans and also in mice. To assess the effect of an immune response to the adenoviral vector in mice, the protocol was tested in immunocompetent mice carrying a mouse melanoma skin tumor. The experiment involved i.v. instead of intratumoral injections of the vector, because the mouse melanoma cells are not infected by the vector, as shown below. Also, only the mfVIIasm immunoconjugate could be tested, because the mouse melanoma cells bind the mfVIIasm immunoconjugate but not the G71-1 immunoconjugate (data not shown). The Fc effector domain of the immunoconjugate for this experiment was derived from a mouse IgG1 immunoglobulin. The results show that i.v. injections of the adenoviral vector encoding the mfVIIasm immunoconjugate results in growth inhibition of a melanoma tumor in immunocompetent mice as well as in SCID mice (Fig. 7).
Figure 7.
Effect of i.v. injections into immunocompetent mice of the adenoviral vector encoding the mfVIIasm immunoconjugate on the growth of a mouse skin melanoma. C57BL/6 mice were injected s.c. with the mouse melanoma line B16F10, and when a skin tumor had grown to the size indicated on day 0 the mice were injected intravenously with 3 × 1010 IP of the vector encoding the mfVIIasm immunoconjugate. The Fc domain of the immunoconjugate was derived from a mouse IgG1 immunoglobulin. The mice were injected intravenously again on day 5 with 1.5 × 1010 IP of the same vector.
Because TF is generally expressed by tumor vascular endothelial cells and also by most tumor cells, a fVIIasm immunoconjugate could mediate a cytolytic immune response not only against human melanomas but also against a broad spectrum of other human tumors. As shown in the study reported here, intratumoral injections of an adenoviral vector encoding the mfVIIasm immunoconjugate into a human tumor appears to be a safe and effective protocol for establishing and maintaining a high blood titer of the immunoconjugate. However, the cells of the injected tumor must be susceptible to infection by the adenovirus and be capable of synthesizing and secreting the encoded immunoconjugate. A panel of human and mouse tumor lines, consisting of human melanoma (LXSN, TF2, and YUsac2), prostate cancer (LnCap), breast cancer (BT20), pancreatic cancer (Colo357), renal cancer (Caki), gastric cancer (MS), and neuroblastoma (A204) lines and mouse melanoma (B16F10) and breast cancer (EMT6) lines were tested as hosts for the adenoviral vector encoding the mfVIIasm immunoconjugate. All of the human tumor lines produced and secreted about the same amount of the immunoconjugate protein (data not shown), indicating that the intratumoral injection protocol could also be used for other types of human solid tumors in addition to melanoma. The mouse tumor lines failed to produce the immunoconjugate protein, probably because the mouse cells were not infected by the adenoviral vector.
Discussion
The cancer immunotherapy protocol described in this report involves intratumoral injections of replication-incompetent adenoviral vectors encoding immunoconjugate molecules targeted to tumor vasculature endothelial cells and tumor cells (Fig. 1). The cells infected by the vectors synthesize the encoded immunoconjugates, which are secreted into the blood and bind to the targeted cells, resulting in a cytolytic immune response against tumors accessible to the bloodborne immunoconjugates. Intratumoral injection of the vectors provides an important safety advantage over i.v. injection (11), because the vector infects predominantly the cells of the injected tumor instead of liver cells (Fig. 2). Many types of human tumor cells can be infected by the vector and can synthesize and secrete the encoded immunoconjugates. Thus, the intratumoral injection route for the vectors should be generally applicable to human solid tumors. If there is no tumor accessible for injection, the vector could be injected into a normal tissue such as muscle.
The therapeutic efficacy of the intratumoral injection protocol was tested in a SCID mouse model of human skin melanoma and metastatic lung melanoma. The tests involved two immunoconjugates containing different targeting domains. (i) One targeting domain is the blood zymogen factor VII (fVII) that binds with high affinity and specificity to the transmembrane receptor tissue factor expressed by endothelial cells of growing blood vessels, including the vessels of the tumor neovasculature, and also by most human tumor cells. A fVII immunoconjugate must compete in vivo with endogenous fVII for binding to tissue factor on the targeted cells. This competition strongly favors the homodimeric immunoconjugate molecule (Fig. 1) over the monomeric endogenous molecule, because the avidity effect of two targeting domains enhances binding to cells expressing multiple copies of tissue factor (11); also, the blood titer is higher for the vector-encoded fVII immunoconjugate than for endogenous fVII. A mouse fVII targeting domain was used for the experiments involving human tumor xenografts growing in SCID mice, because it binds tightly both to human tissue factor on the tumor cells and to mouse tissue factor on the mouse endothelial cells in the tumor vasculature. To prevent initiation of the blood coagulation pathway by the binding of a fVII immunoconjugate to tissue factor, which could cause disseminated intravascular coagulation, a mutation was introduced into the active site of the mouse fVII targeting domain (mfVIIasm). However, the mfVIIasm immunoconjugate might cause bleeding by interfering with the binding of endogenous fVII to tissue factor. No evidence of bleeding was detected in any of the mice injected intratumorally with the vector encoding the mfVIIasm immunoconjugate. Moreover, another study showed that injection of a coagulation-defective human fVII molecule into normal human subjects, in doses ranging from 10 to 400 μg/kg body weight, did not prolong bleeding time.† These results suggest that bleeding should not pose a significant safety problem for the immunotherapy protocol described here. (ii) The second targeting domain is the single-chain Fv antibody G71-1 that binds to a chondroitin sulfate proteoglycan expressed selectively by human melanoma cells (12, 13). The results of the immunotherapy tests showed that intratumoral injections of the vector encoding the mfVIIasm immunoconjugate, either alone or together with the vector encoding the G71-1 immunoconjugate, inhibited growth of the injected skin tumor and also of metastatic lung tumors (Fig. 4 and Table 1). The residual tumor tissue remaining after intratumoral injections of the vectors encoding the immunoconjugates was almost devoid of blood vessels (Fig. 6), indicating that the mfVIIasm immunoconjugate induces a potent cytolytic immune response resulting in extensive destruction of the tumor neovasculature. A fVII immunoconjugate might also be effective in treating other diseases involving neovascularization.
Acknowledgments
We are grateful for the contributions of the following colleagues at Yale University: Dr. Albert Deisseroth, who provided the resources and facilities for producing adenoviral vectors; Dr. Thomas Hughes, who provided the expertise and equipment for the photographs shown in Fig. 2; Gordon Terwilliger, who dissected and examined the mice; and Dr. Michael Bromberg, who provided the human melanoma lines. Support for this study was derived in part from the National Institutes of Health Program Project Grant HL29019.
Abbreviations
- SCID
severe combined immunodeficient
- IP
infectious particles
- IU
infectious units
- GFP
green fluorescent protein
- fVII
factor VII
Footnotes
References
- 1.Brody S, Jaffee H, Han S, Wersto R. Hum Gene Ther. 1994;5:437–447. doi: 10.1089/hum.1994.5.4-437. [DOI] [PubMed] [Google Scholar]
- 2.Addison C, Braciak T, Ralston R, Muller W, Gauldie J, Graham F. Proc Natl Acad Sci USA. 1995;92:8522–8526. doi: 10.1073/pnas.92.18.8522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood M, Perrotte P, Onishi E, Harper M E, Dinney C, Pagliaro L, Wilson D R. Cancer Gene Ther. 1999;4:367–372. doi: 10.1038/sj.cgt.7700090. [DOI] [PubMed] [Google Scholar]
- 4.Herz J, Gerard R D. Proc Natl Acad Sci USA. 1993;90:2812–2816. doi: 10.1073/pnas.90.7.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang D-C, Johnson S A, Carbone D P. Cancer Gene Ther. 1994;1:15–20. [PubMed] [Google Scholar]
- 6.Lin N, Buxton J, Acheson A, Radziejewski C, Maisonpierre P C, Yancopoulous G D, Channon K M, Hale L P, Dewhirst M W, George S E, Peters K G. Proc Natl Acad Sci USA. 1998;95:8829–8834. doi: 10.1073/pnas.95.15.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu Y, Carraher J, Zhang Y, Armstrong J, Lerner J, Rogers W P, Steiner M S. Cancer Gene Ther. 1998;6:64–72. doi: 10.1038/sj.cgt.7700011. [DOI] [PubMed] [Google Scholar]
- 8.Harvey B G, Hackett N R, El-Sawy T, Rosengart T K, Hirschowitz E A, Lieberman M D, Lesser M L, Crystal R G. J Virol. 1999;73:6729–6742. doi: 10.1128/jvi.73.8.6729-6742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Putzer B M, Bramson J L, Addison C L, Hitt M, Siegel P M, Muller W J, Graham F L. Hum Gene Ther. 1998;9:707–718. doi: 10.1089/hum.1998.9.5-707. [DOI] [PubMed] [Google Scholar]
- 10.Joshi U S, Chen Y Q, Kalemkerian G P, Adil M R, Kraut M, Sarkar F H. Cancer Gene Ther. 1998;5:183–191. [PubMed] [Google Scholar]
- 11.Hu Z, Sun Y, Garen A. Proc Natl Acad Sci USA. 1999;96:8161–8166. doi: 10.1073/pnas.96.14.8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai X, Garen A. Proc Natl Acad Sci USA. 1997;94:9261–9266. doi: 10.1073/pnas.94.17.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang B, Chen Y B, Ayalon O, Bender J, Garen A. Proc Natl Acad Sci USA. 1999;96:1627–1632. doi: 10.1073/pnas.96.4.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]