Abstract
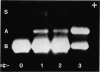
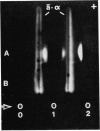
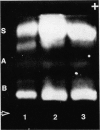
Complementation of beta hexosaminidase A (hex A) deficiency was obtained by Sendai virus-mediated somatic cell hybridization of cultured skin fibroblasts from two unrelated patients with Tay-Sachs disease (TSD) and one patient with Sandhoff-Jatzkewitz disease (SJD). The newly formed hex A was identified by its electrophoretic mobility in three different systems, heat lability, and reactivity with an antiserum against the unique antigenic determinant, alpha of hex A. The percentage of heterokaryons obtained by virus treatment of TSD and SJD fibroblast mixtures showed good correlation with the observed percentage of hex A activity. It is concluded that, in these two forms of GM2 gangliosidosis, beta hexosaminidase deficiency results from two different mutations. All of the current models of beta hexosaminidase structure are compatible with the observed complementation. No complementation was detected in 13 Sendai virus-induced fusions of cultured skin fibroblasts from seven unrelated patients with SJD. The enzyme deficiency in these patients may be due to very similar allelic mutations, not capable of undergoing complementation; or to different structural mutations, all coding for unstable beta hexosaminidase molecules.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholomew W. R., Rattazzi M. C. Immunochemical characterization of human beta-D-N-acetyl hexosaminidase from normal individuals and patients with Tay-Sachs disease. I. Antigenic differences between hexosaminidase A and hexosaminidase B. Int Arch Allergy Appl Immunol. 1974;46(4):512–524. doi: 10.1159/000231154. [DOI] [PubMed] [Google Scholar]
- CATCHESIDE D. G., OVERTON A. Complementation between alleles in heterocaryons. Cold Spring Harb Symp Quant Biol. 1958;23:137–140. doi: 10.1101/sqb.1958.023.01.017. [DOI] [PubMed] [Google Scholar]
- CRICK F. H., ORGEL L. E. THE THEORY OF INTER-ALLELIC COMPLEMENTATION. J Mol Biol. 1964 Jan;8:161–165. doi: 10.1016/s0022-2836(64)80156-x. [DOI] [PubMed] [Google Scholar]
- Carroll M., Robinson D. Immunological properties of N-acetyl-beta-D-glucosaminidase of normal human liver and of GM2-gangliosidosis liver. Biochem J. 1973 Jan;131(1):91–96. doi: 10.1042/bj1310091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galjaard H., Hoogeveen A., de Wit-Verbeek H. A., Reuser A. J., Keijzer W., Westerveld A., Bootsma D. Tay-Sachs and Sandhoff's disease: intergenic complementation after somatic cell hybridization. Exp Cell Res. 1974 Aug;87(2):444–448. doi: 10.1016/0014-4827(74)90515-1. [DOI] [PubMed] [Google Scholar]
- Goldstone A., Konecny P., Koenig H. Lysosomal hydrolases: Conversion of acidic to basic forms by neuraminidase. FEBS Lett. 1971 Feb 12;13(1):68–72. doi: 10.1016/0014-5793(71)80667-1. [DOI] [PubMed] [Google Scholar]
- Harzer K., Sandhoff K., Schall H., Kollmann F. Enzymatische Untersuchungen im Blut von Uberträgern einer Variante der Tay-Sachsschen Erkrankung (Variante O. Klin Wochenschr. 1971 Nov 1;49(21):1189–1191. doi: 10.1007/BF01732464. [DOI] [PubMed] [Google Scholar]
- Ikonne J. U., Rattazzi M. C., Desnick R. J. Characterization of Hex S, the major residual beta hexosaminidase activity in type O Gm2 gangliosidosis (Sandhoff-Jatzkewitz disease). Am J Hum Genet. 1975 Sep;27(5):639–650. [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., Jensen F. C., Steplewski Z. Activation of production of infectious tumor virus SV40 in heterokaryon cultures. Proc Natl Acad Sci U S A. 1967 Jul;58(1):127–133. doi: 10.1073/pnas.58.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEABACK D. H., WALKER P. G. Studies on glucosaminidase. 4. The fluorimetric assay of N-acetyl-beta-glucosaminidase. Biochem J. 1961 Jan;78:151–156. doi: 10.1042/bj0780151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley P. A., Rattazzi M. C., Shows T. B. Human beta-D-N-acetylhexosaminidases A and B: expression and linkage relationships in somatic cell hybrids. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1569–1573. doi: 10.1073/pnas.71.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. T., Mazzotta M. Y., Wan C. C., Orth R., Li S. C. Hydrolysis of Tay-Sachs ganglioside by beta-hexosaminidase A of human liver and urine. J Biol Chem. 1973 Nov 10;248(21):7512–7515. [PubMed] [Google Scholar]
- Miggiano V., Nabholz M., Bodmer W. Hybrids between human leukocytes and a mouse cell line: production and characterization. Wistar Inst Symp Monogr. 1969;9:61–76. [PubMed] [Google Scholar]
- Okada S., McCrea M., O'Brien J. S. Sandhoff's disease (GM 2 gangliosidosis type 2): clinical, chemical, and enzyme studies in five patients. Pediatr Res. 1972 Jul;6(7):606–615. [PubMed] [Google Scholar]
- Okada S., O'Brien J. S. Tay-Sachs disease: generalized absence of a beta-D-N-acetylhexosaminidase component. Science. 1969 Aug 15;165(3894):698–700. doi: 10.1126/science.165.3894.698. [DOI] [PubMed] [Google Scholar]
- Okada S., Veath M. L., Leroy J., O'Brien J. S. Ganglioside GM2 storage diseases: hexosaminidase deficiencies in cultured fibroblasts. Am J Hum Genet. 1971 Jan;23(1):55–61. [PMC free article] [PubMed] [Google Scholar]
- Robinson D., Stirling J. L. N-Acetyl-beta-glucosaminidases in human spleen. Biochem J. 1968 Apr;107(3):321–327. doi: 10.1042/bj1070321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropers H. H., Grzeschik K. H., Bühler E. Complementation after fusion of Sandhoff- and Tay-Sachs fibroblasts. Humangenetik. 1975;26(2):117–121. doi: 10.1007/BF00278438. [DOI] [PubMed] [Google Scholar]
- Sandhoff K., Andreae U., Jatzkewitz H. Deficient hexosaminidase activity in an exceptional case of Tay-Sachs disease with additional storage of kidney globoside in visceral organs. Pathol Eur. 1968;3(2):278–285. [PubMed] [Google Scholar]
- Sandhoff K., Harzer K., Wässle W., Jatzkewitz H. Enzyme alterations and lipid storage in three variants of Tay-Sachs disease. J Neurochem. 1971 Dec;18(12):2469–2489. doi: 10.1111/j.1471-4159.1971.tb00204.x. [DOI] [PubMed] [Google Scholar]
- Sandhoff K., Wässle W. Anreicherung und Charakterisierung zweier Formen der menschlichen N-acetyl- -D-hexosaminidase. Hoppe Seylers Z Physiol Chem. 1971 Aug;352(8):1119–1133. [PubMed] [Google Scholar]
- Srivastava S. K., Awasthi Y. C., Yoshida A., Beutler E. Studies on human beta-D-N-acetylhexosaminidases. I. Purification and properties. J Biol Chem. 1974 Apr 10;249(7):2043–2048. [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Studies on human beta-D-N-acetylhexosaminidases. 3. Biochemical genetics of Tay-Sachs and Sandhoff's diseases. J Biol Chem. 1974 Apr 10;249(7):2054–2057. [PubMed] [Google Scholar]
- Srivastava S. K., Yoshida A., Awasthi Y. C., Beutler E. Studies on human beta-D-N-acetylhexosaminidases. II. Kinetic and structural properties. J Biol Chem. 1974 Apr 10;249(7):2049–2053. [PubMed] [Google Scholar]
- Suzuki Y., Jacob J. C., Suzuki K., Kutty K. M., Suzuki K. Gm2-gangliosidosis with total hexosaminidase deficiency. Neurology. 1971 Apr;21(4):313–328. doi: 10.1212/wnl.21.4.313. [DOI] [PubMed] [Google Scholar]
- Tallman J. F., Brady R. O., Quirk J. M., Villalba M., Gal A. E. Isolation and relationship of human hexosaminidases. J Biol Chem. 1974 Jun 10;249(11):3489–3499. [PubMed] [Google Scholar]
- Thomas G. H., Taylor H. A., Miller C. S., Axelman J., Migeon B. R. Genetic complementation after fusion of Tay-Sachs and Sandhoff cells. Nature. 1974 Aug 16;250(467):580–582. doi: 10.1038/250580a0. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]
- Wenger D. A., Okada S., O'Brien J. S. Studies on the substrate specificity of hexosaminidase A and B from liver. Arch Biochem Biophys. 1972 Nov;153(1):116–129. doi: 10.1016/0003-9861(72)90427-4. [DOI] [PubMed] [Google Scholar]