Abstract
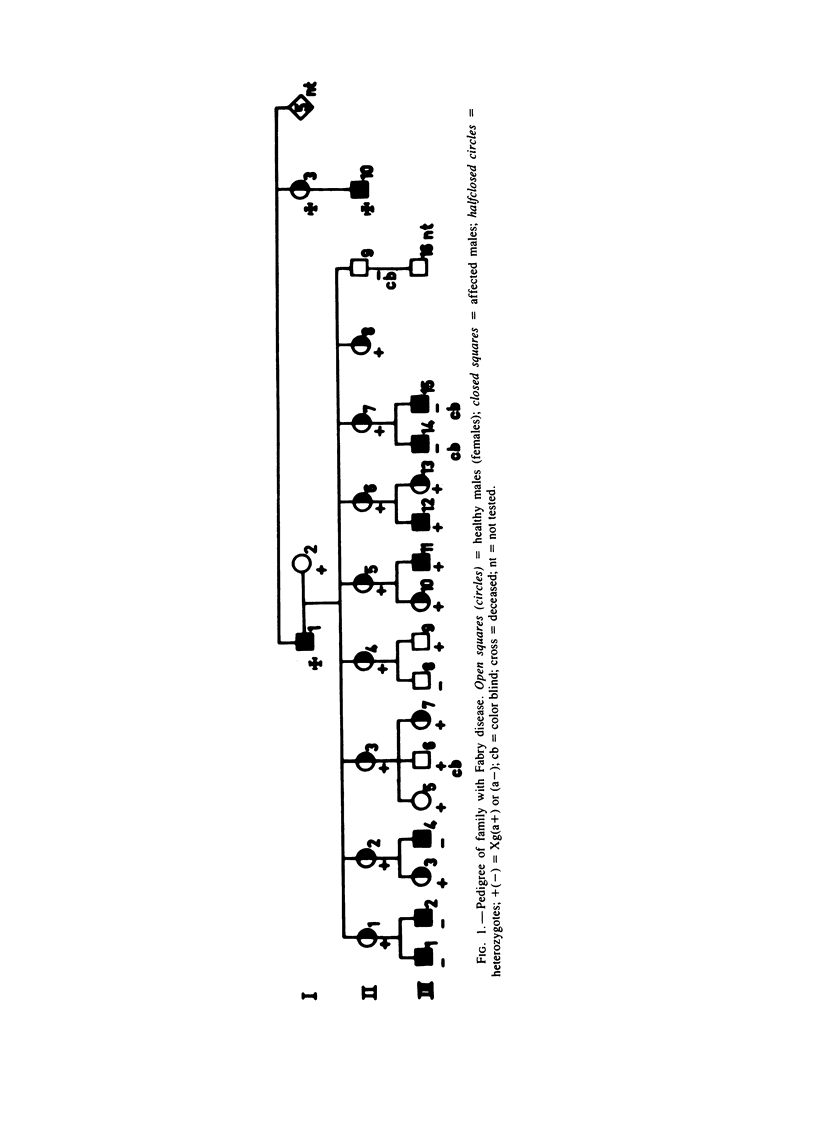
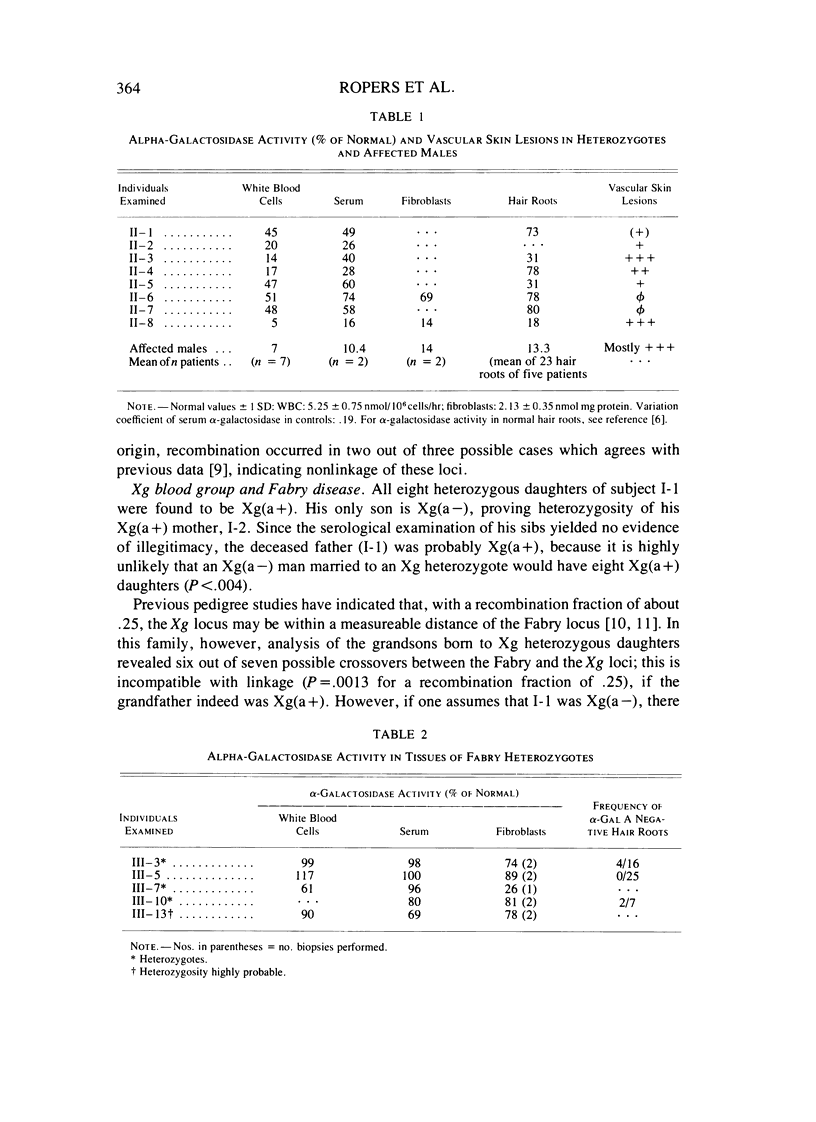
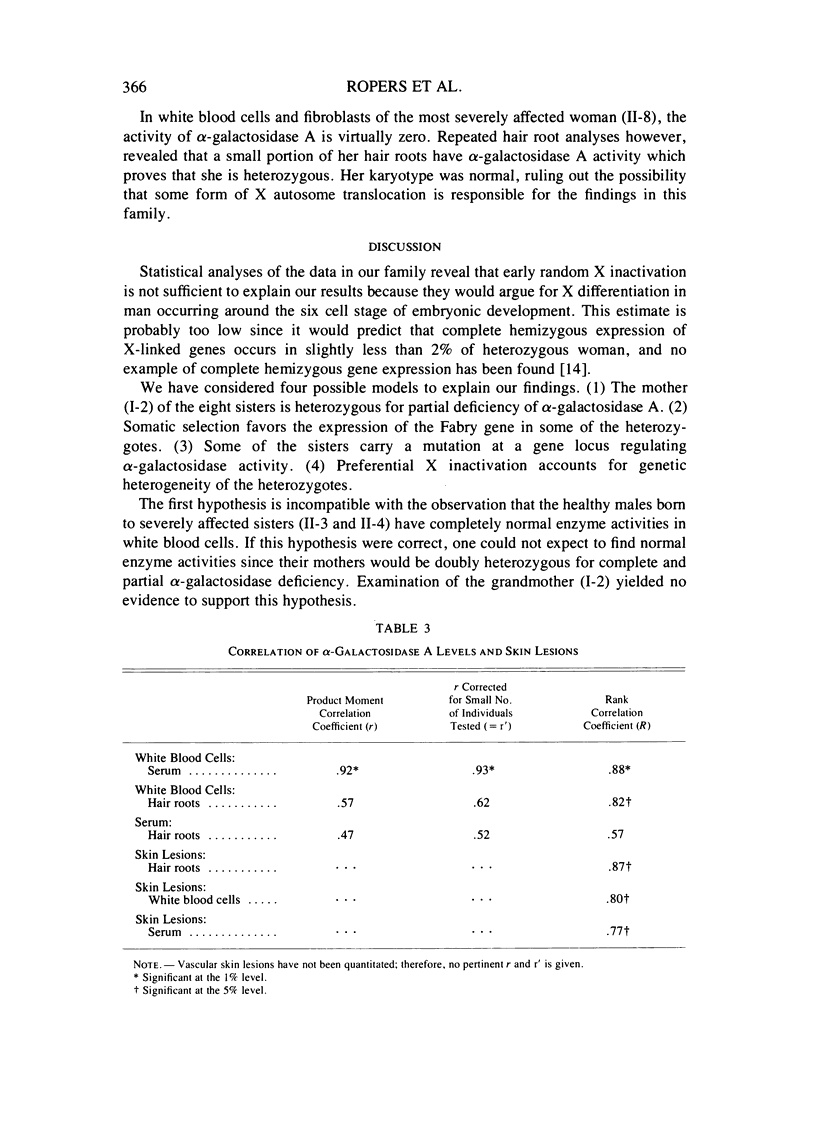
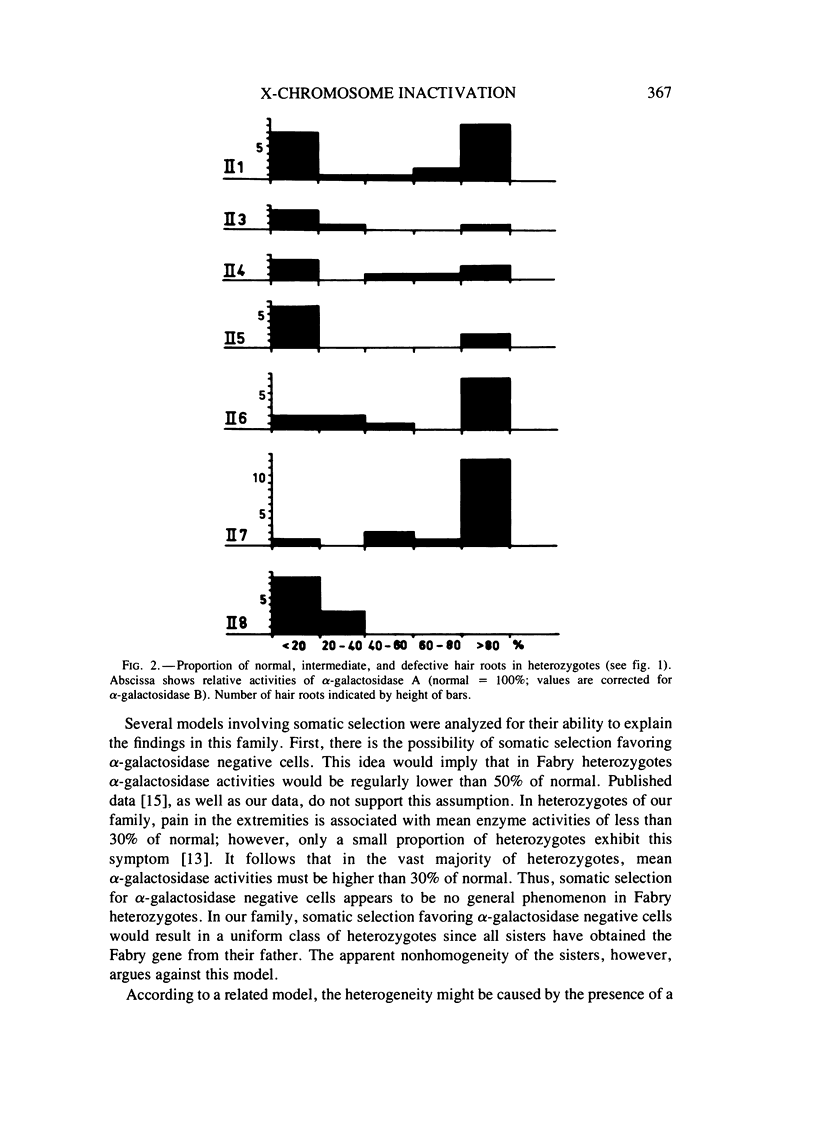
Severe clinical signs of Fabry disease were observed in four of eight heterozygous daughters of a male patient. Activities of alpha-galactosidase A in serum, white blood cells, and hair roots of the manifesting carriers were markedly lower than 50% of normal. These findings are not easy to interpret in terms of random X inactivation alone; several alternative models including nonrandom (preferential) X inactivation are discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini R. J., DeMars R. Mosaicism of peripheral blood lymphocyte populations in females heterozygous for the Lesch-Nyhan mutation. Biochem Genet. 1974 May;11(5):397–411. doi: 10.1007/BF00486413. [DOI] [PubMed] [Google Scholar]
- Grimm T., Wienker T. F., Ropers H. H. Fabry's disease: heterozygote detection by hair root analysis. Hum Genet. 1976 Jun 29;32(3):329–334. doi: 10.1007/BF00295824. [DOI] [PubMed] [Google Scholar]
- Johnston A. W., Frost P., Spaeth G. L., Renwick J. H. Linkage relationships of the angiokeratoma (Fabry) locus. Ann Hum Genet. 1969 May;32(4):369–374. doi: 10.1111/j.1469-1809.1969.tb00088.x. [DOI] [PubMed] [Google Scholar]
- Langenbeck U., Grimm T., Rüdiger H. W., Passarge E. Heterozygote tests and genetic counseling in maple syrup urine disease: an application of Baye's theorem. Humangenetik. 1975;27(4):315–322. doi: 10.1007/BF00278424. [DOI] [PubMed] [Google Scholar]
- Lusis A. J., Paigen K. Genetic determination of the alpha-galactosidase developmental program in mice. Cell. 1975 Nov;6(3):371–378. doi: 10.1016/0092-8674(75)90186-5. [DOI] [PubMed] [Google Scholar]
- McDonald J. A., Kelley W. N. Lesch-Nyhan syndrome: absence of the mutant enzyme in erythrocytes of a heterozygote for both normal and mutant hypoxanthine-guanine phosphoribosyl transferase. Biochem Genet. 1972 Feb;6(1):21–26. doi: 10.1007/BF00485961. [DOI] [PubMed] [Google Scholar]
- Moser H., Emery A. E. The manifesting carrier in Duchenne muscular dystrophy. Clin Genet. 1974;5(4):271–284. doi: 10.1111/j.1399-0004.1974.tb01694.x. [DOI] [PubMed] [Google Scholar]
- Nesbit M. N. X chromosome inactivation mosaicism in the mouse. Dev Biol. 1971 Oct;26(2):252–263. doi: 10.1016/0012-1606(71)90125-4. [DOI] [PubMed] [Google Scholar]
- Nesbitt M. N., Gartler S. M. The applications of genetic mosaicism to developmental problems. Annu Rev Genet. 1971;5:143–162. doi: 10.1146/annurev.ge.05.120171.001043. [DOI] [PubMed] [Google Scholar]
- Opitz J. M., Stiles F. C., Wise D., Race R. R., Sanger R., Von Gemmingen G. R., Kierland R. R., Cross E. G., De Groot W. P. The Genetics of Angiokeratoma Corporis Diffusum (Fabry's Disease) and Its Linkage Relations with the Xg Locus. Am J Hum Genet. 1965 Jul;17(4):325–342. [PMC free article] [PubMed] [Google Scholar]
- Romeo G., D'Urso M., Pisacane A., Blum E., De Falco A., Ruffilli A. Residual activity of alpha-galactosidase A in Fabry's disease. Biochem Genet. 1975 Oct;13(9-10):615–628. doi: 10.1007/BF00484919. [DOI] [PubMed] [Google Scholar]
- Ropers H. H., Grzeschik K. H., Bühler E. Complementation after fusion of Sandhoff- and Tay-Sachs fibroblasts. Humangenetik. 1975;26(2):117–121. doi: 10.1007/BF00278438. [DOI] [PubMed] [Google Scholar]


