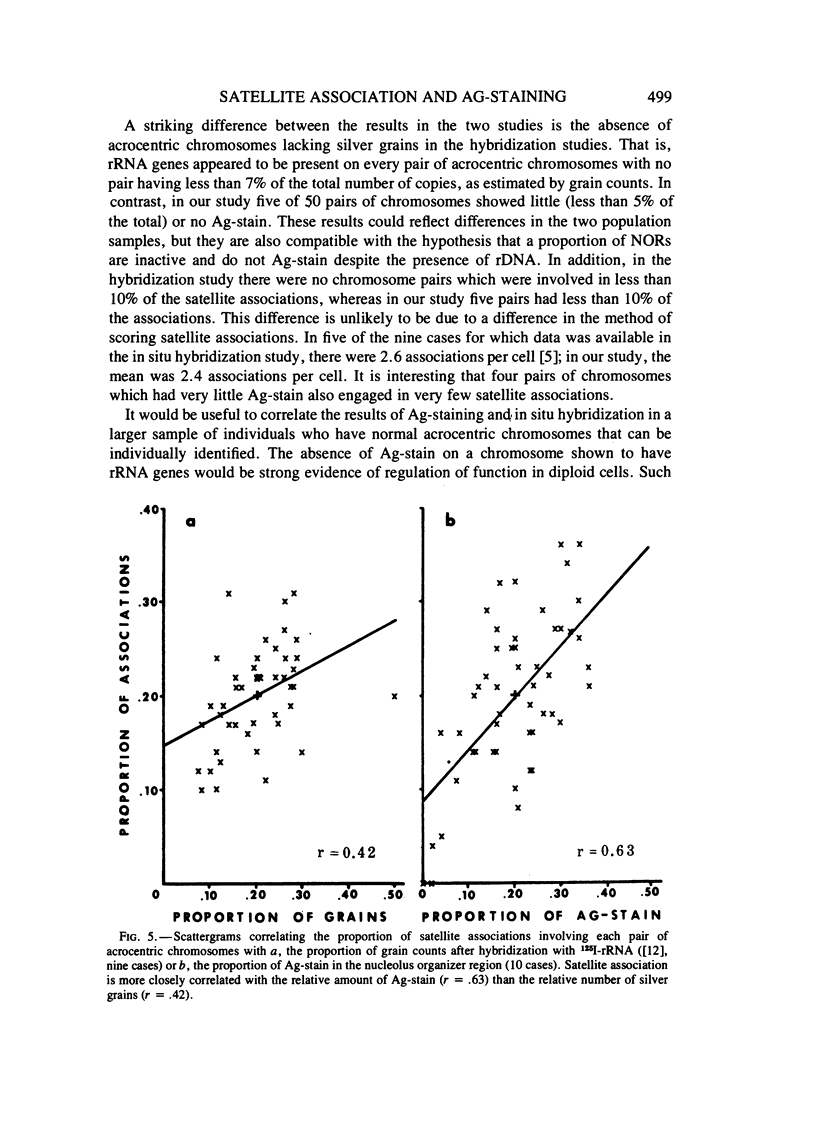
Abstract
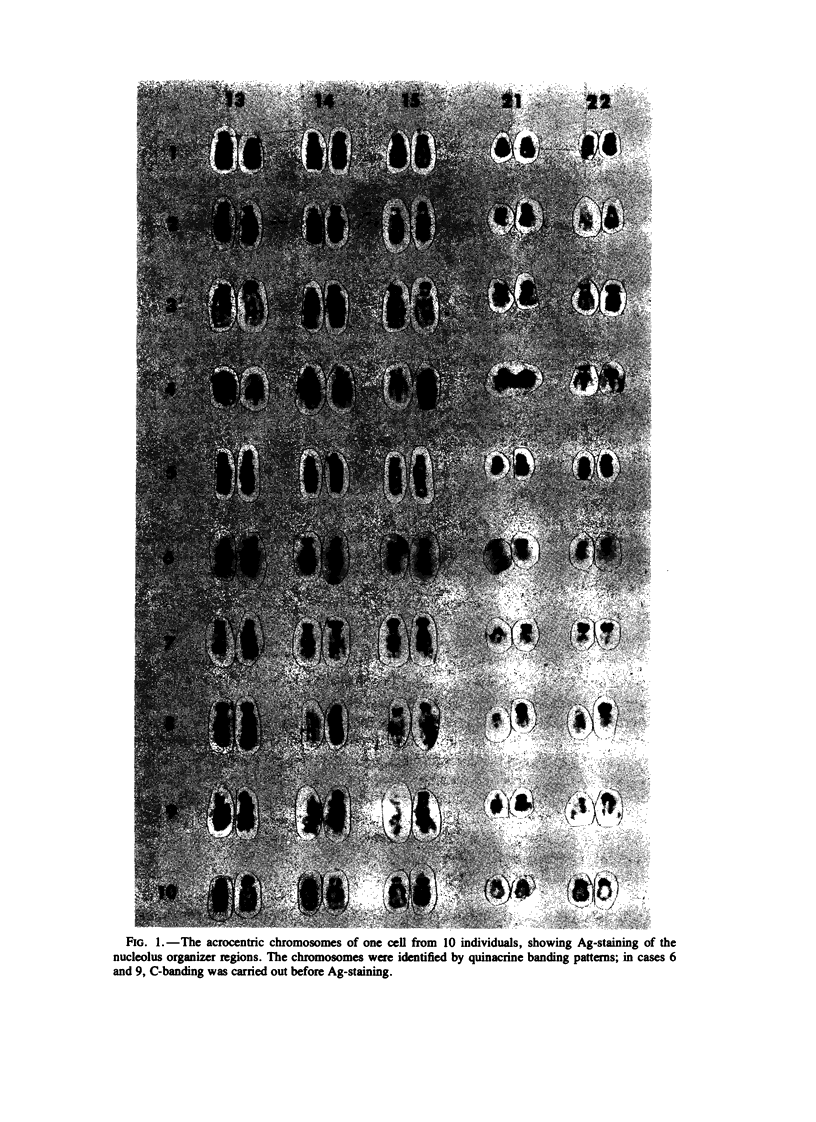
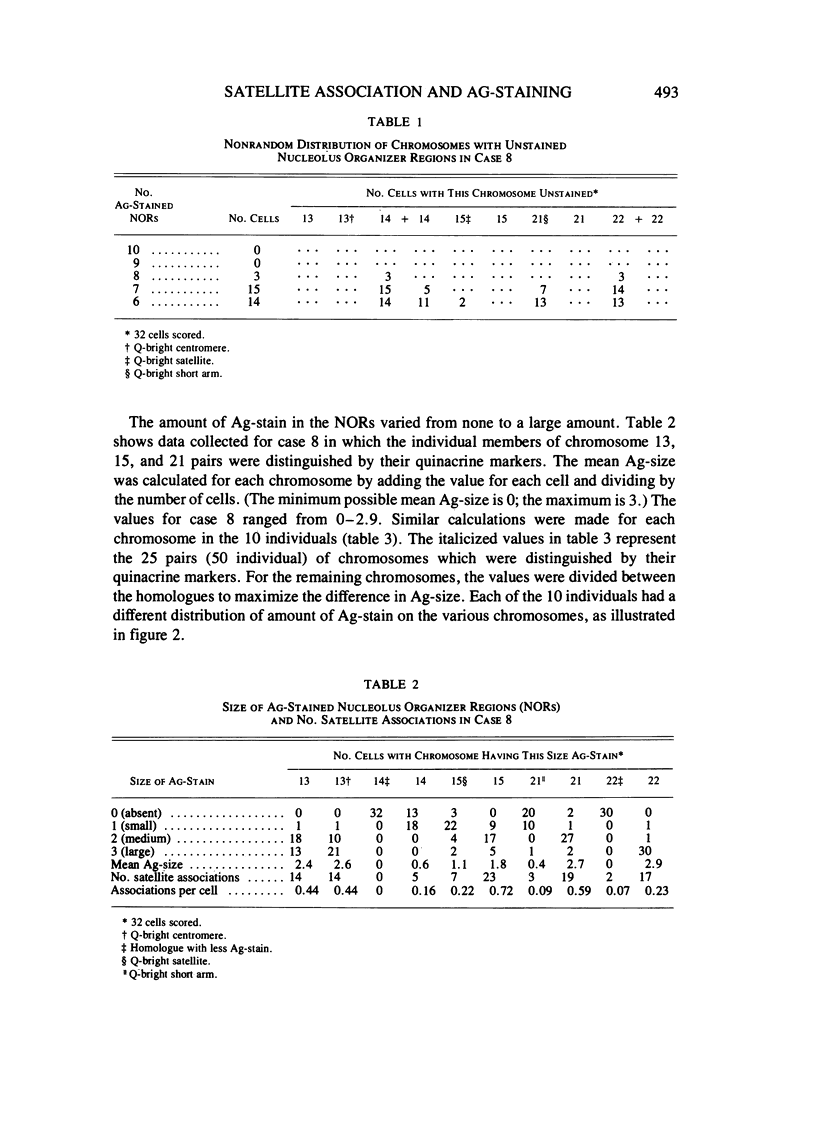
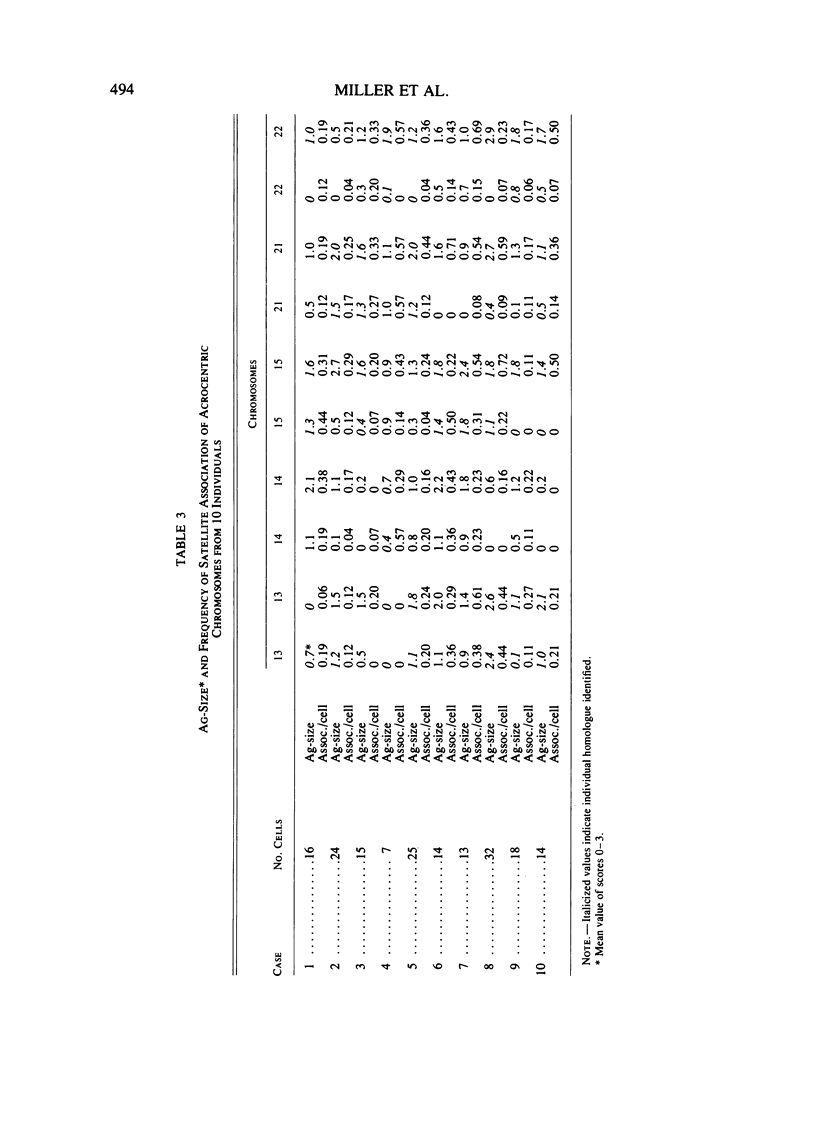
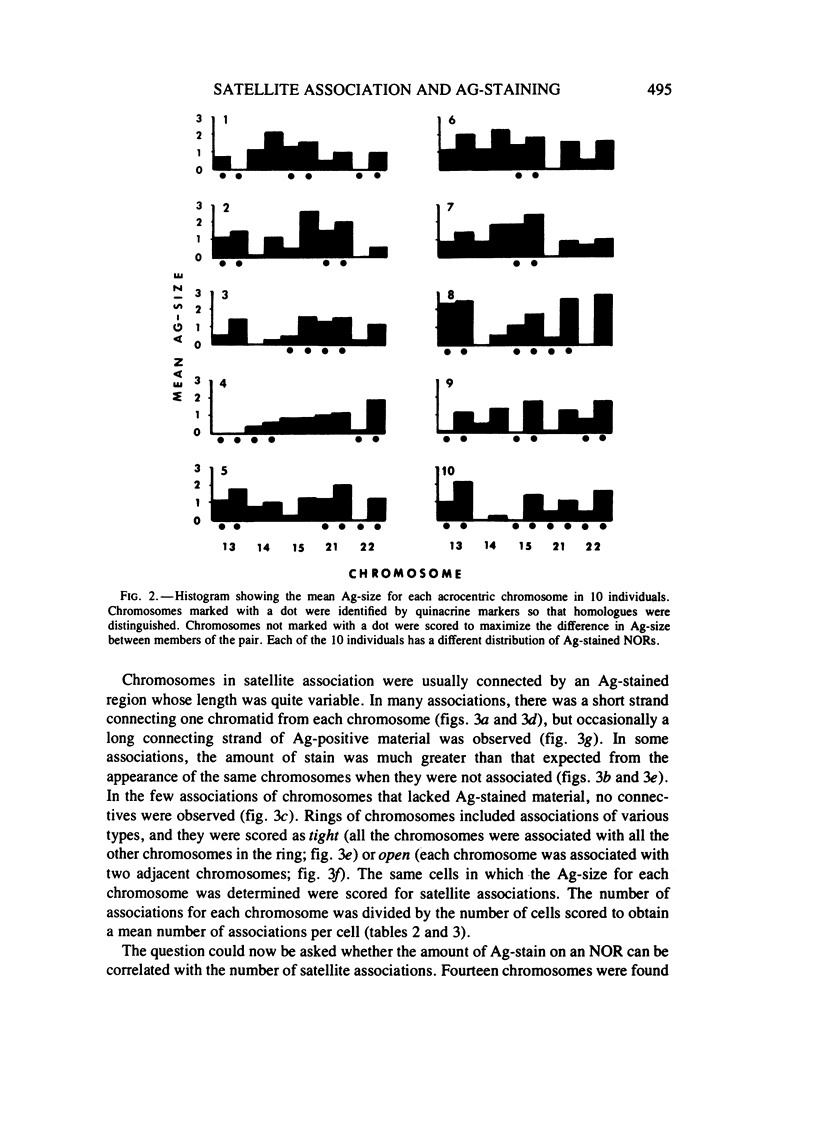
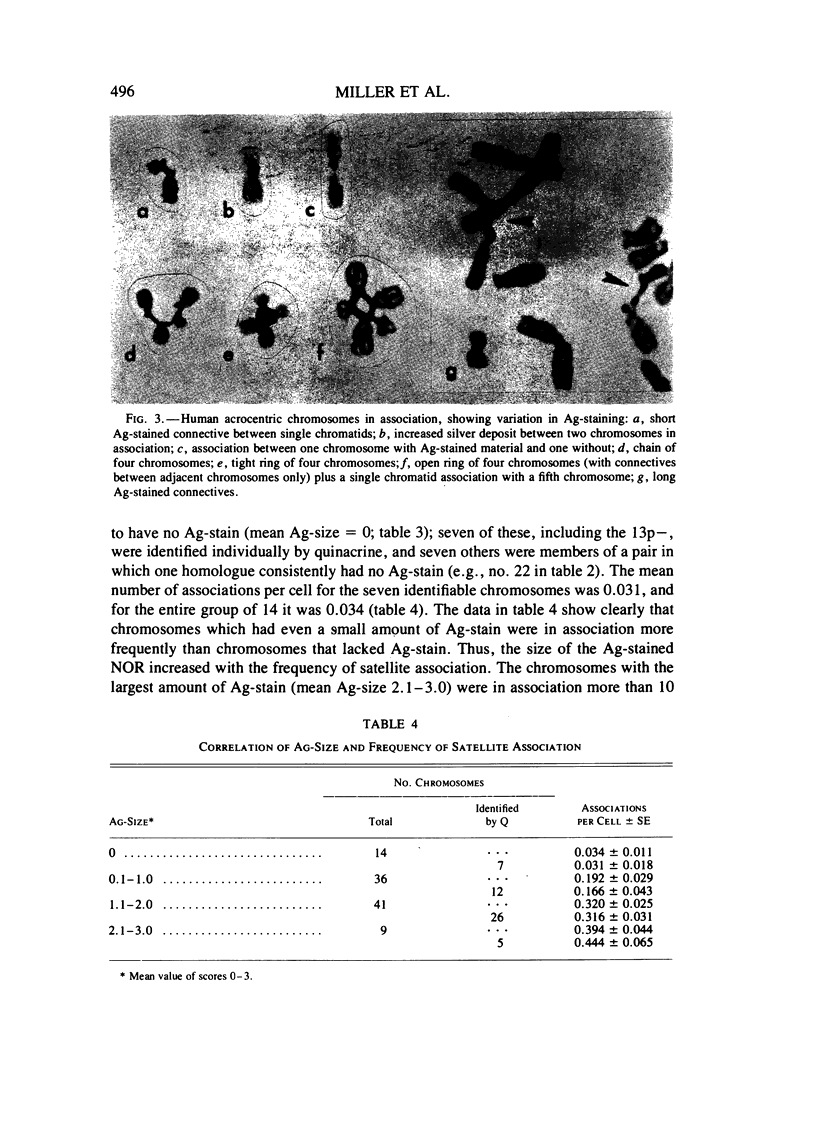
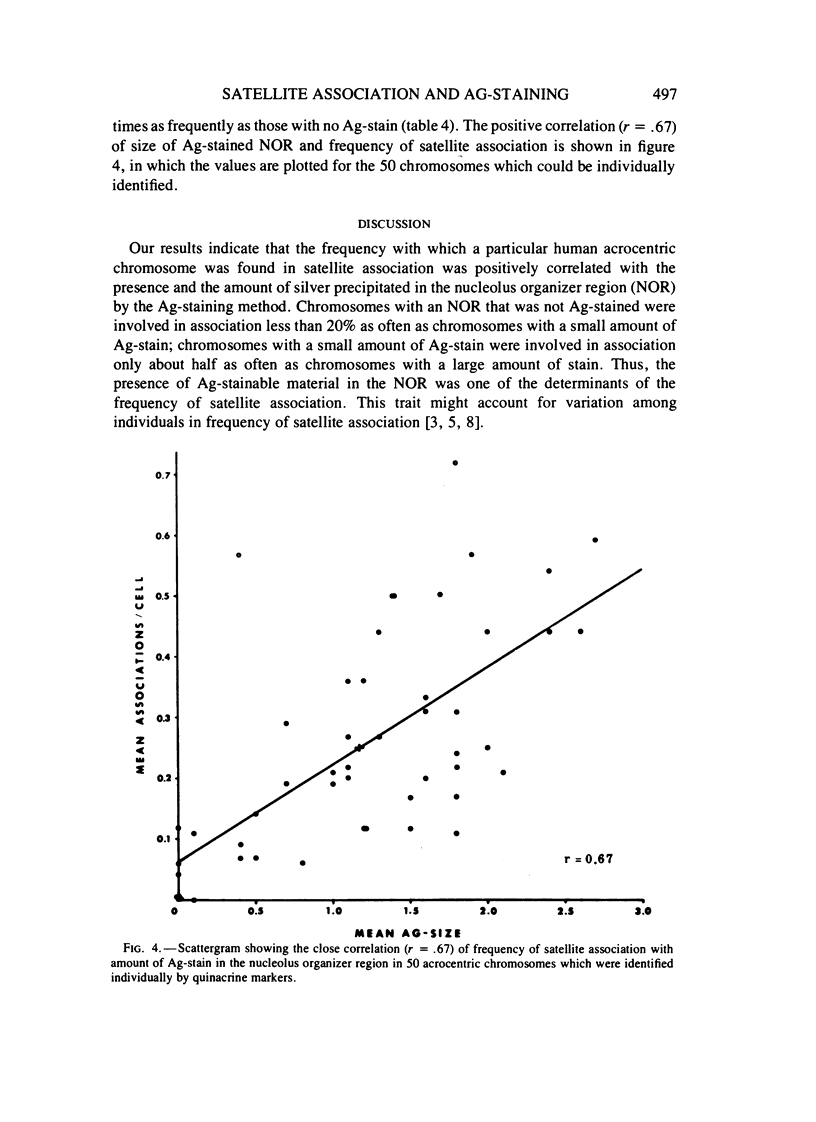
Methaphase chromosomes from karyotypically normal adult humans (three males, six females) and one male with a 13p - chromosome were stained by quinacrine and then by the Ag-AS silver staining method to reveal nucleolus organizer regions (NORs). Each person had a characteristic number of Ag-stained chromosomes per cell, always fewer than 10. Determination of the mean Ag-size of each chromosome showed that each of the 10 individuals had a unique distribution of Ag-stain. Within each individual, there was some variation from cell to cell in the number of acrocentric chromosomes that were Ag-stained; this was not random, and the same chromosomes (those that had at most a small amount of Ag-stain) tended to be unstained in every cell. Satellite associations were scored on the same cells. Chromosomes that had no Ag-stain were involved in satellite association less than 20% as often as those that had some Ag-stain. Chromosomes that had a small amount of Ag-stain were involved in association about 50% as often as those that had a large amount of stain. Regression analysis of the 50 (of a total of 100) acrocentric chromosomes which could be individually identified by quinacrine markers showed that the frequency with which a chromosome was involved in satellite association was strongly correlated with the amount of Ag-stained material in the NOR.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cassidy D. M., Blackler A. W. Repression of nucleolar organizer activity in an interspecific hybrid of the genus Xenopus. Dev Biol. 1974 Nov;41(1):84–96. doi: 10.1016/0012-1606(74)90285-1. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Talavera A., Basilico C., Miller O. J. Suppression of production of mouse 28S ribosomal RNA in mouse-human hybrids segregating mouse chromosomes. Proc Natl Acad Sci U S A. 1977 Feb;74(2):694–697. doi: 10.1073/pnas.74.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittes H., Krone W., Bross K., Schmid M., Vogel W. Biochemical and cytogenetic studies on the nucleolus organizing regions (NOR) of man. II. A family with the 15/21 translocation. Humangenetik. 1975;26(1):47–59. doi: 10.1007/BF00280284. [DOI] [PubMed] [Google Scholar]
- FERGUSON-SMITH M. A., HANDMAKER S. D. Observations on the satellited human chromosomes. Lancet. 1961 Mar 25;1(7178):638–640. doi: 10.1016/s0140-6736(61)91655-5. [DOI] [PubMed] [Google Scholar]
- FERGUSON-SMITH M. A. THE SITES OF NUCLEOLUS FORMATION IN HUMAN PACHYTENE CHROMOSOMES. Cytogenetics. 1964;3:124–134. doi: 10.1159/000129803. [DOI] [PubMed] [Google Scholar]
- Goodpasture C., Bloom S. E., Hsu T. C., Arrighi F. E. Human nucleolus organizers: the satellites or the stalks? Am J Hum Genet. 1976 Nov;28(6):559–566. [PMC free article] [PubMed] [Google Scholar]
- Goodpasture C., Bloom S. E. Visualization of nucleolar organizer regions im mammalian chromosomes using silver staining. Chromosoma. 1975 Nov 20;53(1):37–50. doi: 10.1007/BF00329389. [DOI] [PubMed] [Google Scholar]
- Henderson A. S., Warburton D., Atwood K. C. Letter: Ribosomal DNA connectives between human acrocentric chromosomes. Nature. 1973 Sep 14;245(5420):95–97. doi: 10.1038/245095b0. [DOI] [PubMed] [Google Scholar]
- Henderson A. S., Warburton D., Atwood K. C. Location of ribosomal DNA in the human chromosome complement. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3394–3398. doi: 10.1073/pnas.69.11.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T., Reeder R. H. Preferential transcription of Xenopus laevis ribosomal RNA in interspecies hybrids between Xenopus laevis and Xenopus mulleri. J Mol Biol. 1973 Oct 25;80(2):217–228. doi: 10.1016/0022-2836(73)90168-x. [DOI] [PubMed] [Google Scholar]
- Howell W. M., Denton T. E. Negative silver staining in A-T and satellite DNA-rich regions of human chromosomes. Chromosoma. 1976 Aug 17;57(2):165–169. doi: 10.1007/BF00292914. [DOI] [PubMed] [Google Scholar]
- Hsu T. C., Spirito S. E., Pardue M. L. Distribution of 18+28S ribosomal genes in mammalian genomes. Chromosoma. 1975 Nov 20;53(1):25–36. doi: 10.1007/BF00329388. [DOI] [PubMed] [Google Scholar]
- Jacobs P. A., Mayer M., Morton N. E. Acrocentric chromosome associations in man. Am J Hum Genet. 1976 Nov;28(6):567–576. [PMC free article] [PubMed] [Google Scholar]
- Marshall C. J. Synthesis of ribosomal RNA in synkaryons and heterokaryons formed between human and rodent cells. J Cell Sci. 1975 Mar;17(3):307–325. doi: 10.1242/jcs.17.3.307. [DOI] [PubMed] [Google Scholar]
- Mattei J. F., Ayme S., Mattei M. G., Gouvernet J., Giraud F. Quantitative and qualitative study of acrocentric associations in 109 normal subjects. Hum Genet. 1976 Oct 28;34(2):185–194. doi: 10.1007/BF00278887. [DOI] [PubMed] [Google Scholar]
- Miller D. A., Dev V. G., Tantravahi R., Miller O. J. Suppression of human nucleolus organizer activity in mouse-human somatic hybrid cells. Exp Cell Res. 1976 Sep;101(2):235–243. doi: 10.1016/0014-4827(76)90373-6. [DOI] [PubMed] [Google Scholar]
- Miller D. A., Tantravahi R., Dev V. G., Miller O. J. Q- and C-band chromosome markers in inbred strains of Mus musculus. Genetics. 1976 Sep;84(1):67–75. doi: 10.1093/genetics/84.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller O. J., Miller D. A., Dev V. G., Tantravahi R., Croce C. M. Expression of human and suppression of mouse nucleolus organizer activity in mouse-human somatic cell hybrids. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4531–4535. doi: 10.1073/pnas.73.12.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S. R., Lubs H. A. Non-random association of human acrocentric chromosomes. Humangenetik. 1971;13(2):157–159. doi: 10.1007/BF00295797. [DOI] [PubMed] [Google Scholar]
- Schmid M., Krone W., Vogel W. On the relationship between the frequency of association and the nucleolar constriction of individual acrocentric chromosomes. Humangenetik. 1974;23(4):267–277. doi: 10.1007/BF00272510. [DOI] [PubMed] [Google Scholar]
- Tantravahi R., Miller D. A., Dev V. G., Miller O. J. Detection of nucleolus organizer regions in chromosomes of human, chimpanzee, gorilla, orangutan and gibbon. Chromosoma. 1976 Jun 30;56(1):15–27. doi: 10.1007/BF00293725. [DOI] [PubMed] [Google Scholar]
- Warburton D., Atwood K. C., Henderson A. S. Variation in the number of genes for rRNA among human acrocentric chromosomes: correlation with frequency of satellite association. Cytogenet Cell Genet. 1976;17(4):221–230. doi: 10.1159/000130715. [DOI] [PubMed] [Google Scholar]
- Zang K. D., Back E. Quantitative studies on the arrangement of human metaphase chromosomes. I. Individual features in the association pattern of the acrocentric chromosomes of normal males and females. Cytogenetics. 1968;7(6):455–470. [PubMed] [Google Scholar]
- Zankl H., Zang K. D. Quantitative studies on the arrangement of human metaphase chromosomes. IV. The association frequency of human acrocentric marker chromosomes. Humangenetik. 1974;23(4):259–265. doi: 10.1007/BF00272509. [DOI] [PubMed] [Google Scholar]