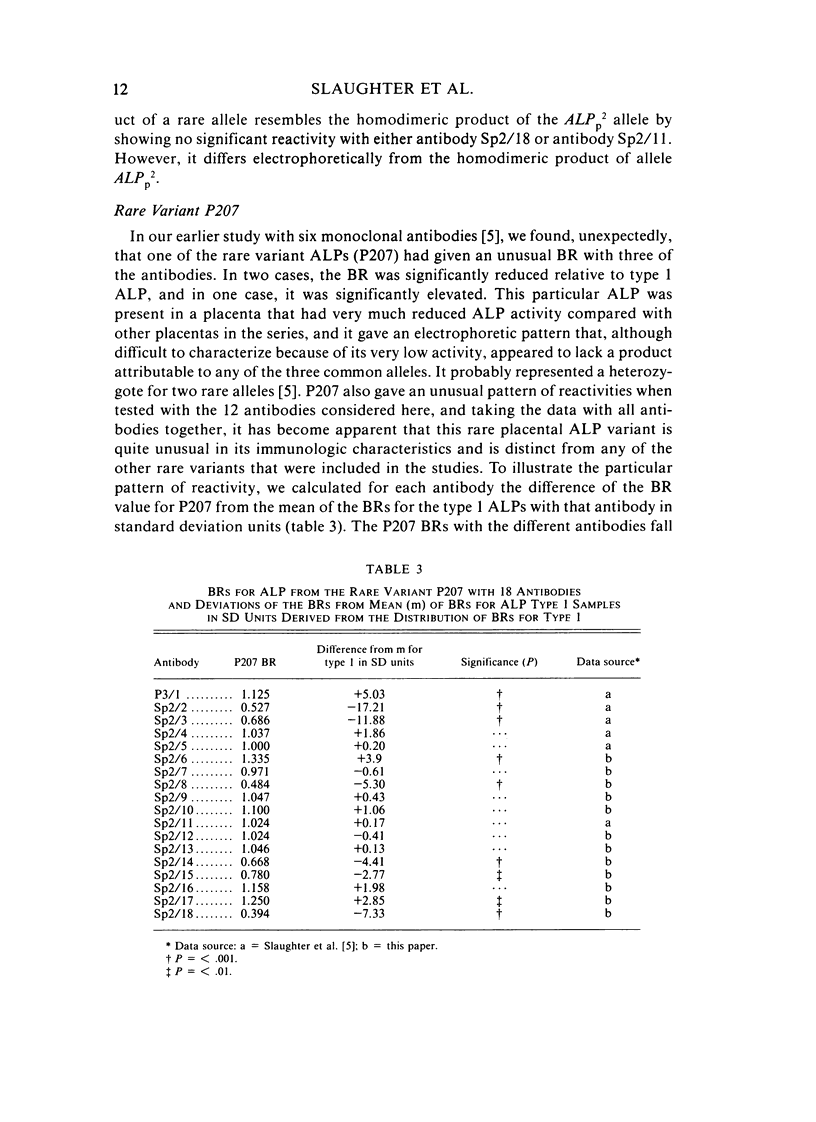
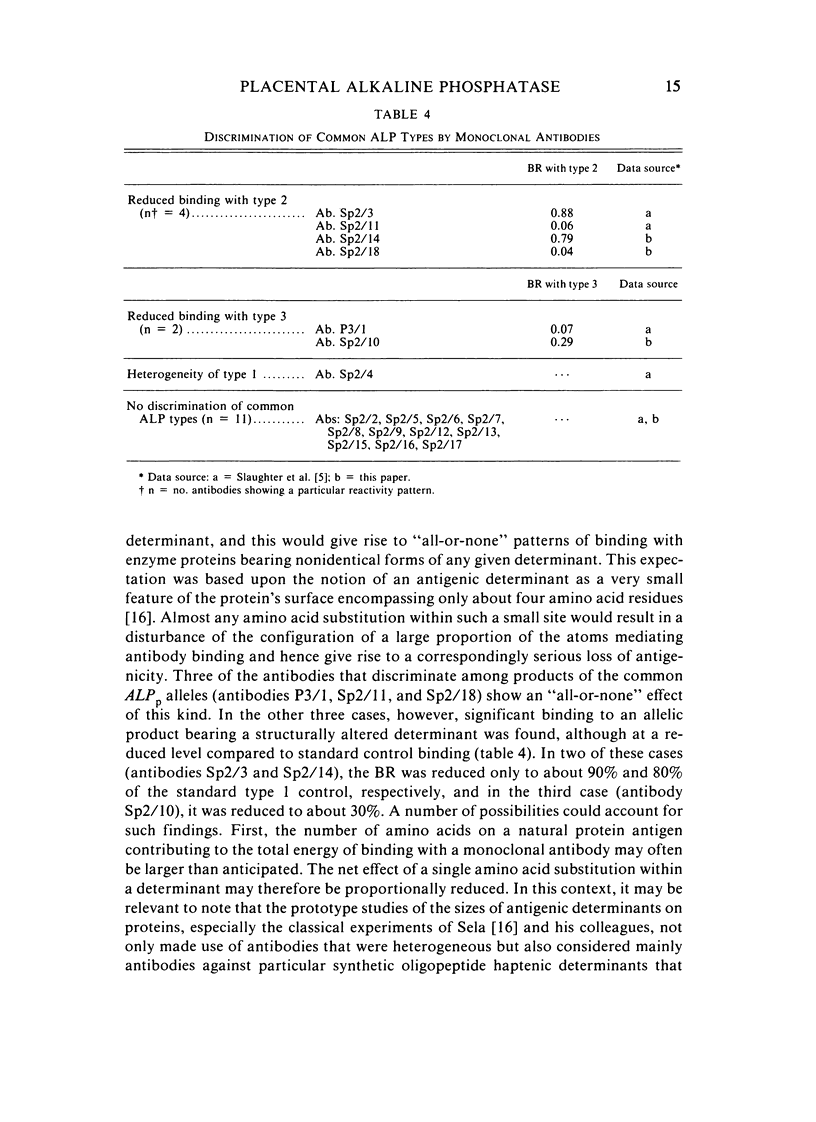
Abstract
Eighteen monoclonal antibodies were produced by the mouse hybridoma method using purified placental alkaline phosphatase (ALP) as antigen. The ability of the various antibodies to discriminate among allelic variants of the enzyme was tested using a large panel of placental ALPs that had been typed electrophoretically. The panel included sets of samples of each of the six common polymorphic phenotypes as well as a series of rare variants. The reactivity of each antibody with each placental ALP (binding ratio) was determined relative to a single standard placental ALP (type 1) in a quantitative binding assay. The findings for six of the antibodies have already been reported. The results on the other 12 antibodies are presented here, and the combined data on the total series of 18 antibodies are analyzed and discussed. Six of the 18 antibodies showed significantly reduced binding to one or another of the products of the three common alleles. In three cases, the discrimination was reflected by essentially "all-or-none" binding reactions. In the other three cases, the binding differences were less marked but could be demonstrated by quantitative comparisons of the binding ratios. Quantitative binding ratio comparisons also enabled heterozygotes to be differentiated from homozygotes in each case. Some of the antibodies showed reduced binding with certain of the rare variant ALP electrophoretic phenotypes. It is estimated that at a minimum this unselected series of 18 antibodies is directed to at least nine different antigenic determinants on the surface of the placental ALP molecule. The results illustrate the power of monoclonal antibodies to discriminate among allelic variants of enzymes.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cancro M. P., Gerhard W., Klinman N. R. The diversity of the influenza-specific primary B-cell repertoire in BALB/c mice. J Exp Med. 1978 Mar 1;147(3):776–787. doi: 10.1084/jem.147.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffino P., Laskov R., Scharff M. D. Immunoglobulin production: method for quantitatively detecting variant myeloma cells. Science. 1970 Jan 9;167(3915):186–188. doi: 10.1126/science.167.3915.186. [DOI] [PubMed] [Google Scholar]
- Donald L. J., Robson E. B. Rare variants of placental alkaline phosphatase. Ann Hum Genet. 1974 Jan;37(3):303–313. doi: 10.1111/j.1469-1809.1974.tb01837.x. [DOI] [PubMed] [Google Scholar]
- Gerhard W. The analysis of the monoclonal immune response to influenza virus. II. The antigenicity of the viral hemagglutinin. J Exp Med. 1976 Oct 1;144(4):985–995. doi: 10.1084/jem.144.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard W., Webster R. G. Antigenic drift in influenza A viruses. I. Selection and characterization of antigenic variants of A/PR/8/34 (HON1) influenza virus with monoclonal antibodies. J Exp Med. 1978 Aug 1;148(2):383–392. doi: 10.1084/jem.148.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolin K. J., Slaughter C. A., Harris H. Electrophoresis of enzyme--monoclonal antibody complexes: studies of human placental alkaline phosphatase polymorphism. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5061–5065. doi: 10.1073/pnas.78.8.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolin K. J., Wray L. K., Slaughter C. A., Harris H. A monoclonal antibody that reacts with nonallelic enzyme glycoproteins. Science. 1982 Apr 2;216(4541):59–61. doi: 10.1126/science.6175022. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Mulivor R. A., Plotkin L. I., Harris H. Differential inhibition of the products of the human alkaline phosphatase loci. Ann Hum Genet. 1978 Jul;42(1):1–13. doi: 10.1111/j.1469-1809.1978.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Potter M., Pumphrey J. G., Walters J. L. Growth of primary plasmacytomas in the mineral oil-conditioned peritoneal environment. J Natl Cancer Inst. 1972 Jul;49(1):305–308. [PubMed] [Google Scholar]
- Robson E. B., Harris H. Further studies on the genetics of placental alkaline phosphatase. Ann Hum Genet. 1967 Jan;30(3):219–232. doi: 10.1111/j.1469-1809.1967.tb00023.x. [DOI] [PubMed] [Google Scholar]
- Robson E. B., Harris H. Genetics of the alkaline phosphatase polymorphism of the human placenta. Nature. 1965 Sep 18;207(5003):1257–1259. doi: 10.1038/2071257a0. [DOI] [PubMed] [Google Scholar]
- Sela M. Antigenicity: some molecular aspects. Science. 1969 Dec 12;166(3911):1365–1374. doi: 10.1126/science.166.3911.1365. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Slaughter C. A., Coseo M. C., Cancro M. P., Harris H. Detection of enzyme polymorphism by using monoclonal antibodies. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1124–1128. doi: 10.1073/pnas.78.2.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]