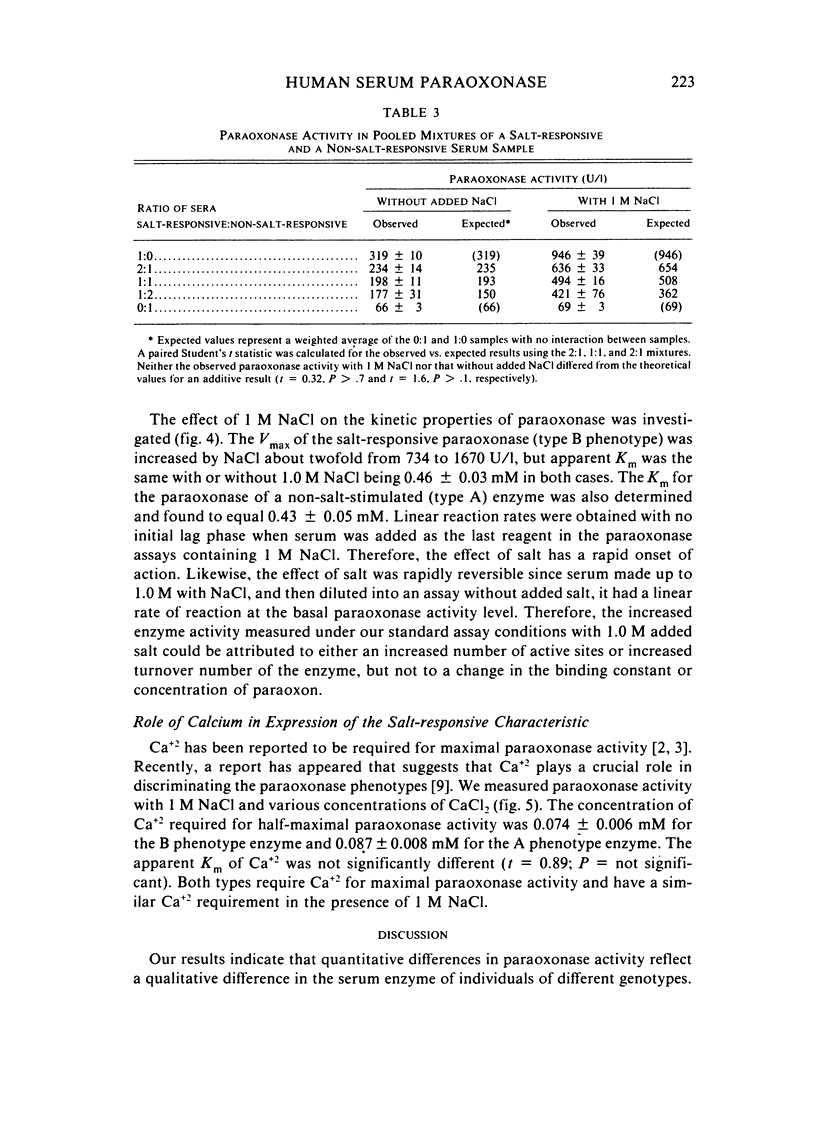
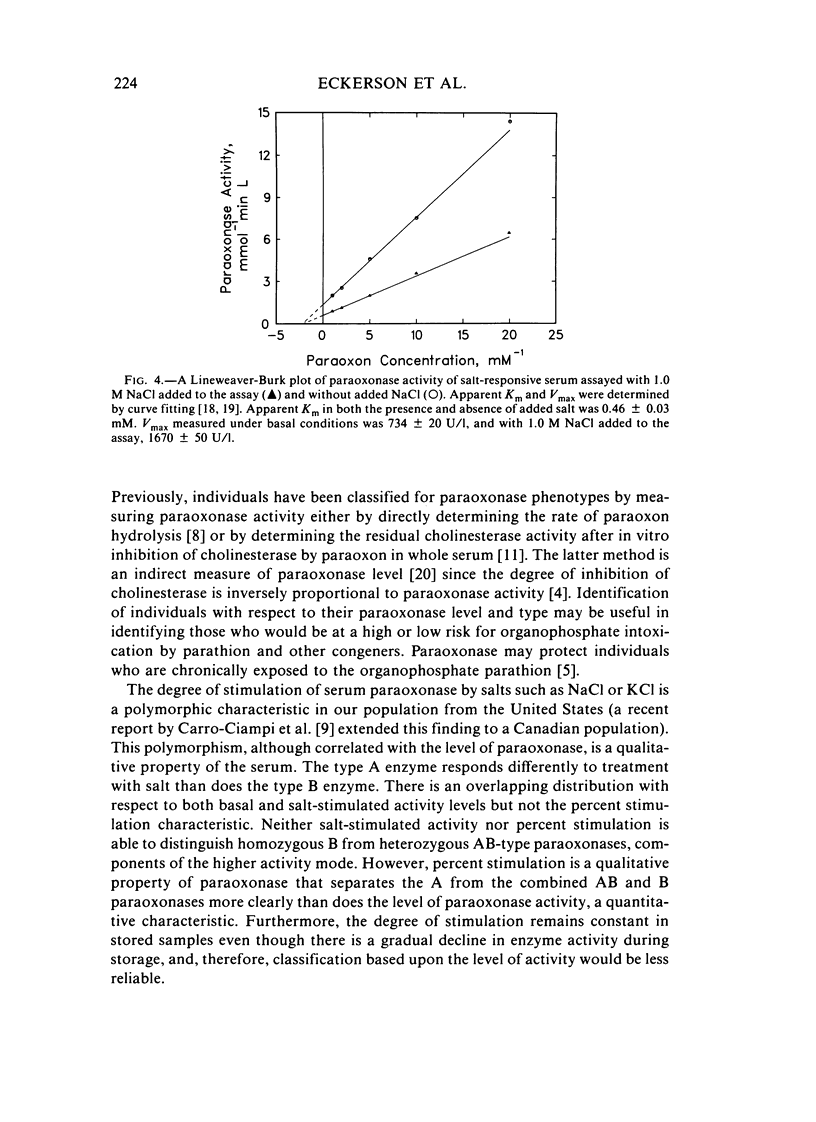
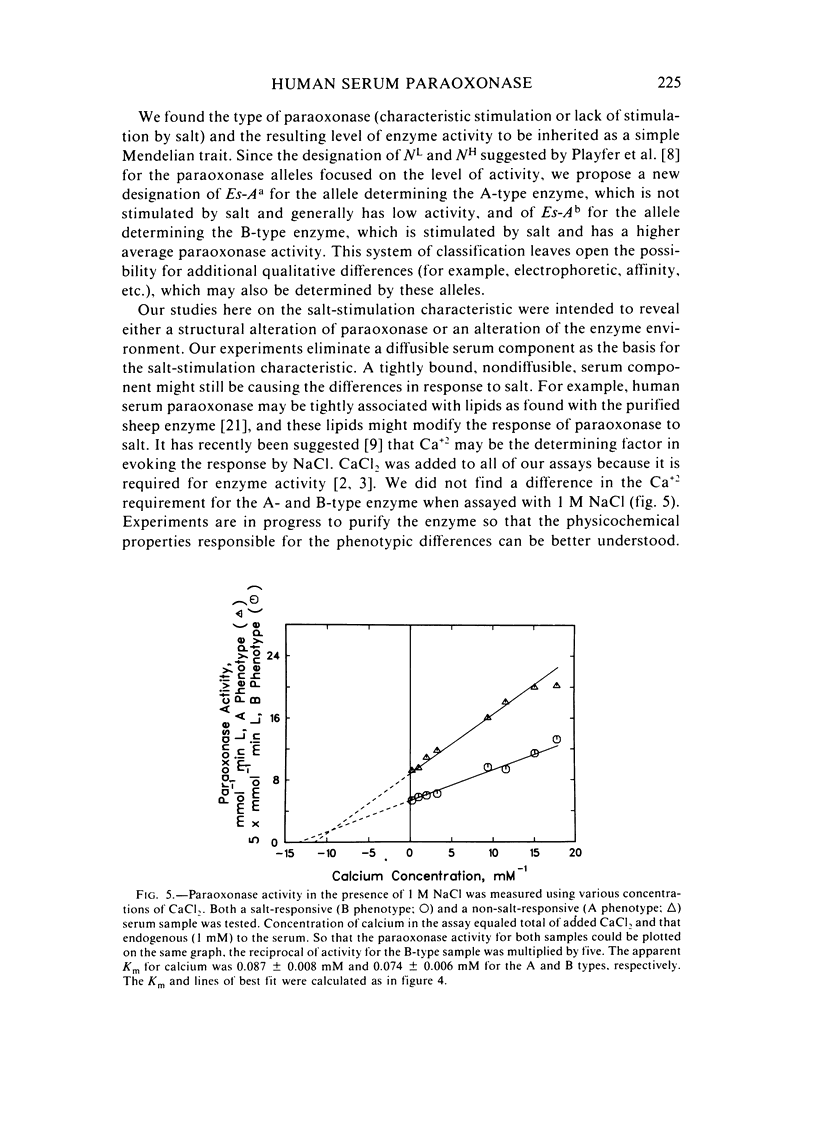
Abstract
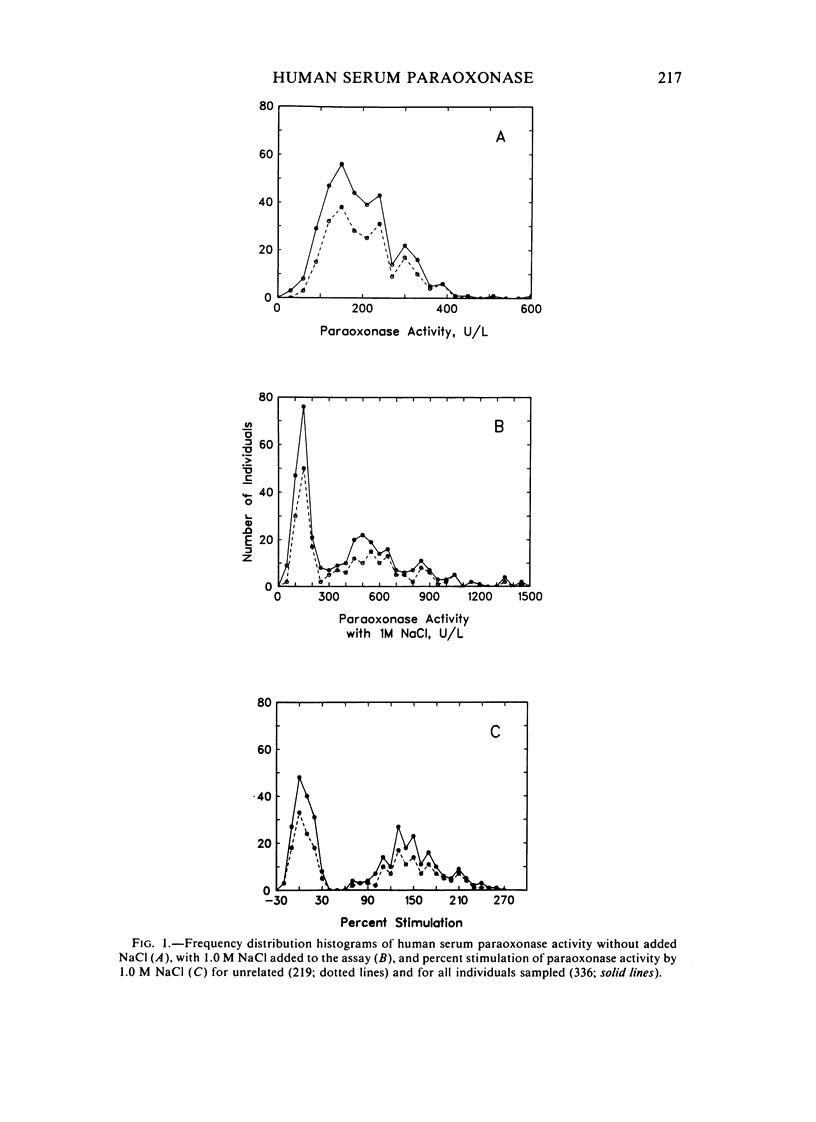
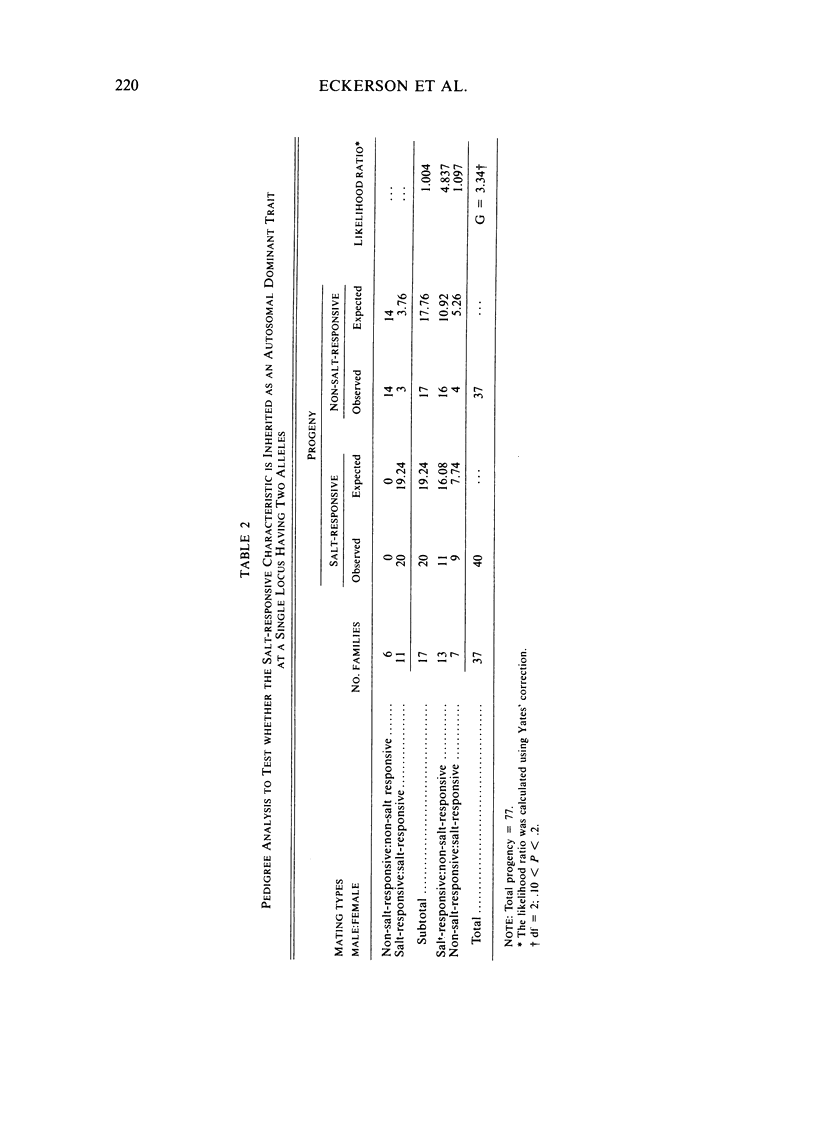
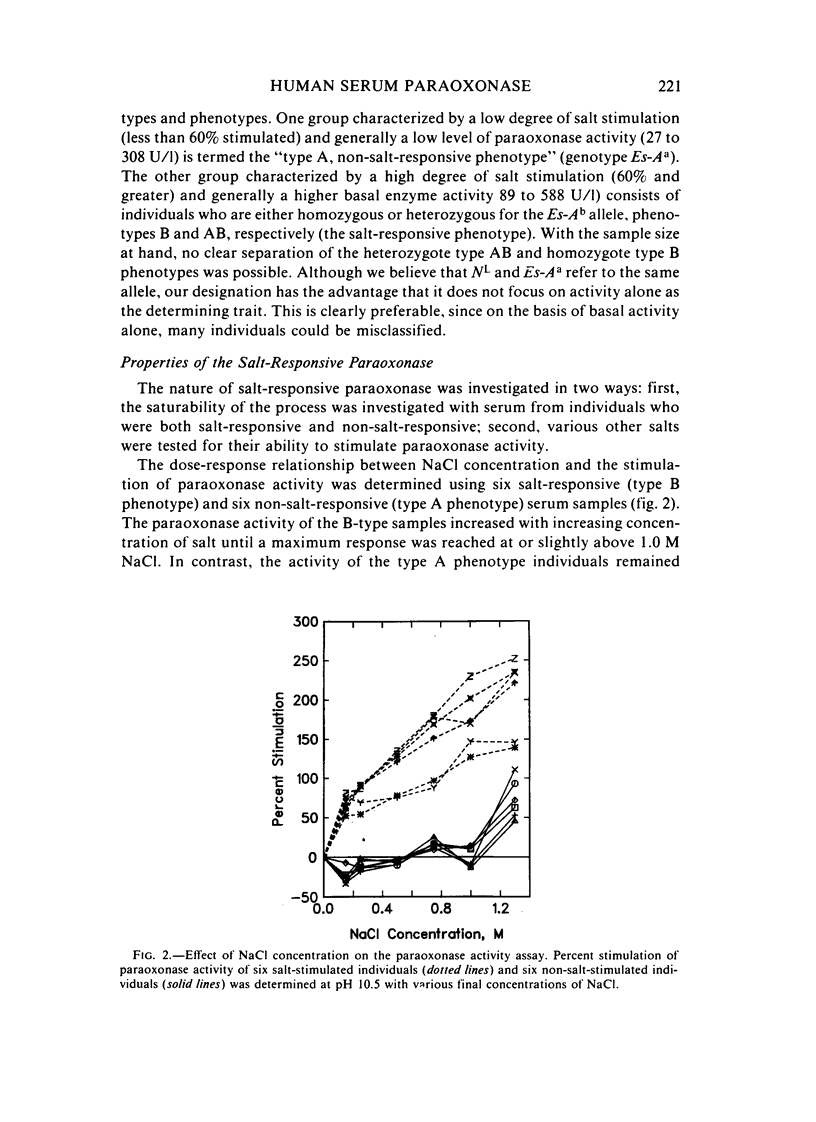
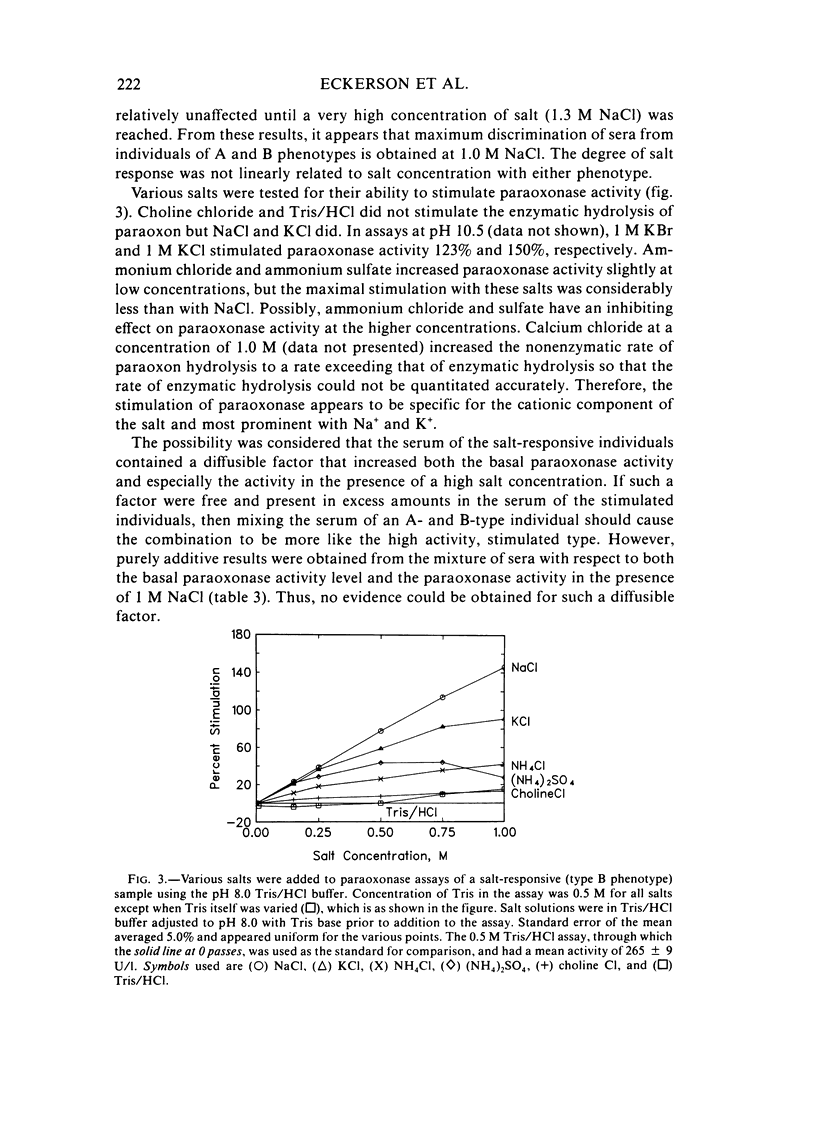
A method for identifying two human serum paraoxonase phenotypes in vitro has been developed based upon the effect of NaCl upon paraoxonase activity. In a sample population of 336 individuals from the United States, 53.9% of the samples had serum paraoxonase that was highly stimulated (60%-257% above the control activity) by 1 M NaCl (salt-responsive), whereas the activity of the remaining samples was not salt-responsive (-23%-35%). The degree of stimulation was consistent and reproducible in frozen samples collected from an individual over a two-year period. Pedigree studies with 37 families indicate that the salt-responsive characteristic is inherited as a simple autosomal, Mendelian trait. Although the salt-responsive individuals on the average had a higher level of activity when assayed without added salt (basal activity) than did the non-salt-responsive individuals, there was considerable overlap in the basal paraoxonase activities. The quantitative polymorphism in serum paraoxonase activity observed in other laboratories is associated with a qualitative difference, quite possibly due to two distinct isozymic forms of the enzyme. A new designation for these alleles is proposed, and some preliminary studies on the molecular basis of the polymorphism are reported.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N. Serum esterases. II. An enzyme hydrolysing diethyl p-nitrophenyl phosphate (E600) and its identity with the A-esterase of mammalian sera. Biochem J. 1953 Jan;53(1):117–124. doi: 10.1042/bj0530117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. Computer programmes for processing enzyme kinetic data. Nature. 1963 May 4;198:463–465. doi: 10.1038/198463a0. [DOI] [PubMed] [Google Scholar]
- Carro-Ciampi G., Kadar D., Kalow W. Distribution of serum paraoxon hydrolyzing activities in a Canadian population. Can J Physiol Pharmacol. 1981 Aug;59(8):904–907. doi: 10.1139/y81-138. [DOI] [PubMed] [Google Scholar]
- ERDOS E. G., BOGGS L. E. Hydrolysis of paraoxon in mammalian blood. Nature. 1961 May 20;190:716–717. doi: 10.1038/190716a0. [DOI] [PubMed] [Google Scholar]
- Eiberg H., Mohr J. Genetics of paraoxonase. Ann Hum Genet. 1981 Oct;45(Pt 4):323–330. doi: 10.1111/j.1469-1809.1981.tb00345.x. [DOI] [PubMed] [Google Scholar]
- Flügel M., Geldmacher-von Mallinckrodt M. Zur Kinetik des Paraoxon-spaltenden Enzyms im menschlichen Serum (EC 3.1.1.2). Klin Wochenschr. 1978 Sep 15;56(18):911–916. doi: 10.1007/BF01489217. [DOI] [PubMed] [Google Scholar]
- Geldmacher-v Mallinckrodt M., Hommel G., Dumbach J. On the genetics of the human serum paraoxonase (EC 3.1.1.2). Hum Genet. 1979 Sep;50(3):313–326. doi: 10.1007/BF00399398. [DOI] [PubMed] [Google Scholar]
- Geldmacher-von Mallinck, Baumgartner W., Pétenyi M., Burgis H., Lindorf H. H., Metzner H. Korrelation zwischen der unterschidlichen Vergiftbarkeit der Serum-Cholinesterase durch E 600 und der Aktivität des E 600-spaltenden Enzym-Systems in menschlichen Seren. Hoppe Seylers Z Physiol Chem. 1972 Feb;353(2):217–220. [PubMed] [Google Scholar]
- Geldmacher-von Mallinck, Lindorf H. H., Petenyi M., Flügel M., Fischer T., Hiller T. Genetisch determinierter Polymorphismus der menschlichen Serum-Paraoxonase (EC 3.1.1.2. Humangenetik. 1973;17(4):331–335. [PubMed] [Google Scholar]
- Geldmacher-von Mallinckrodt M., Pétényi M., Flügel M., Burgis H., Dietzel B., Metzner H., Nirschl H., Renner O. Zur Spezifitt der menschlichen Serum-Paraoxonase. Hoppe Seylers Z Physiol Chem. 1973 Mar;354(3):337–340. [PubMed] [Google Scholar]
- Krisch K. Enzymatische Hydrolyse von Diäthyl-p-nitrophenylphosphat (E 600) durch menschlichen Serum. Z Klin Chem Klin Biochem. 1968 Jan;6(1):41–45. [PubMed] [Google Scholar]
- MAIN A. R. The purification of the enzyme hydrolysing diethyl p-nitrophenyl phosphate (paraoxon) in sheep serum. Biochem J. 1960 Jan;74:10–20. doi: 10.1042/bj0740010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playfer J. R., Eze L. C., Bullen M. F., Evans D. A. Genetic polymorphism and interethnic variability of plasma paroxonase activity. J Med Genet. 1976 Oct;13(5):337–342. doi: 10.1136/jmg.13.5.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson N. E. Serum arylesterase levels of activity in twins and their parents. Am J Hum Genet. 1971 Jul;23(4):375–382. [PMC free article] [PubMed] [Google Scholar]
- Skrinjarić-Spoljar M., Reiner E. Hydrolysis of diethyl-p-nitrophenylphosphate and ethyl-p-nitrophenylethylphosphonate by human sera. Biochim Biophys Acta. 1968 Sep 3;165(2):289–292. doi: 10.1016/0304-4165(68)90059-7. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zech R., Zürcher K. Organophosphate splitting serum enzymes in different mammals. Comp Biochem Physiol B. 1974 Jul 15;48(3):427–433. doi: 10.1016/0305-0491(74)90277-6. [DOI] [PubMed] [Google Scholar]


