Abstract
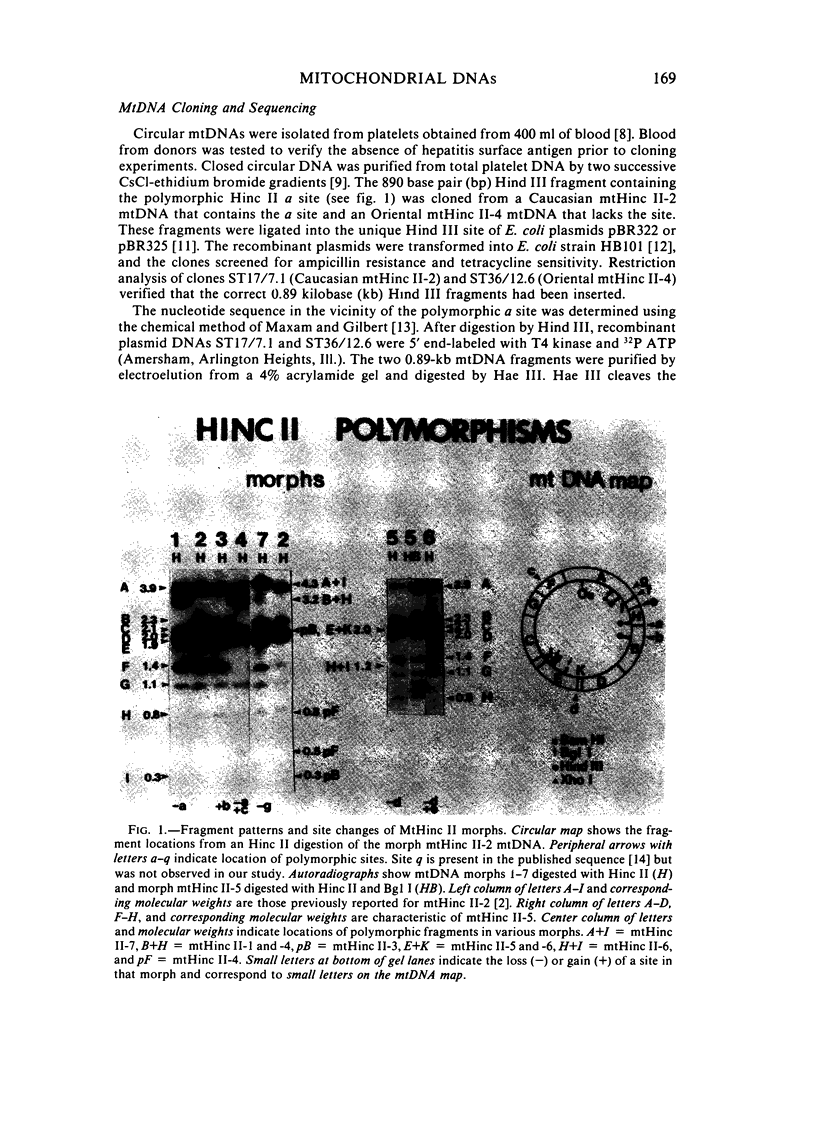
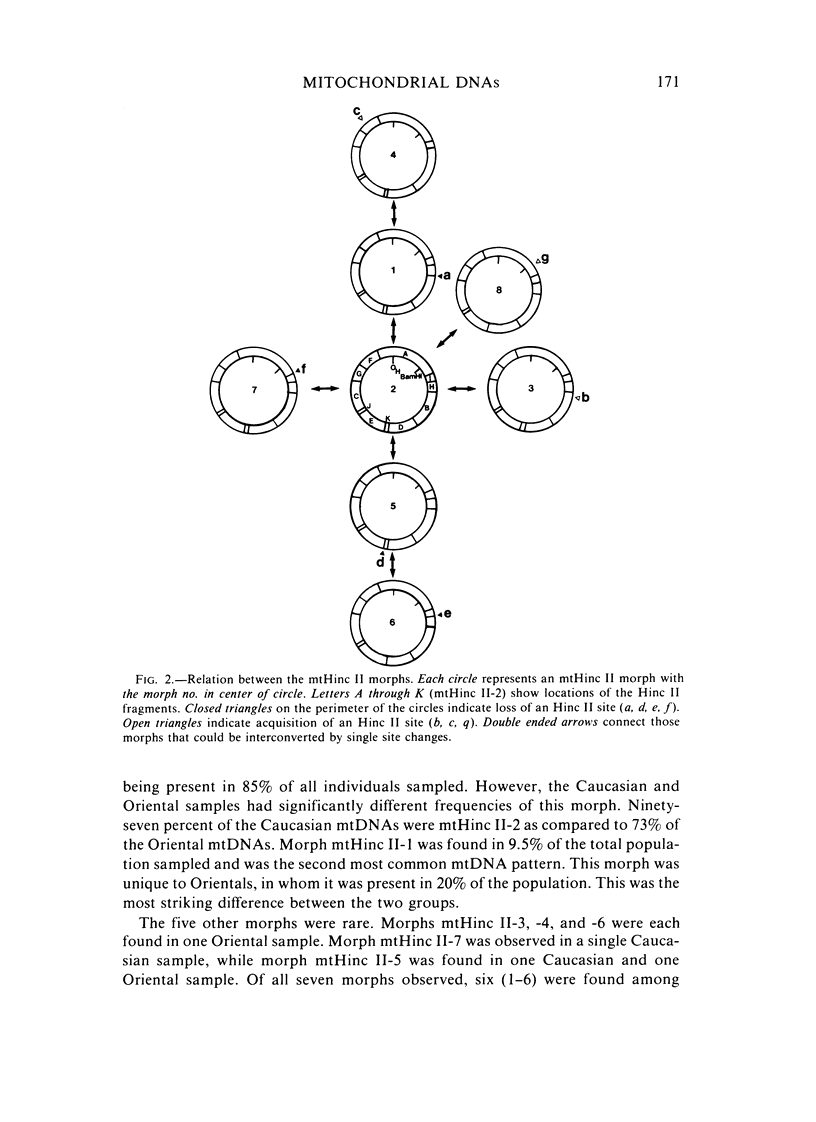
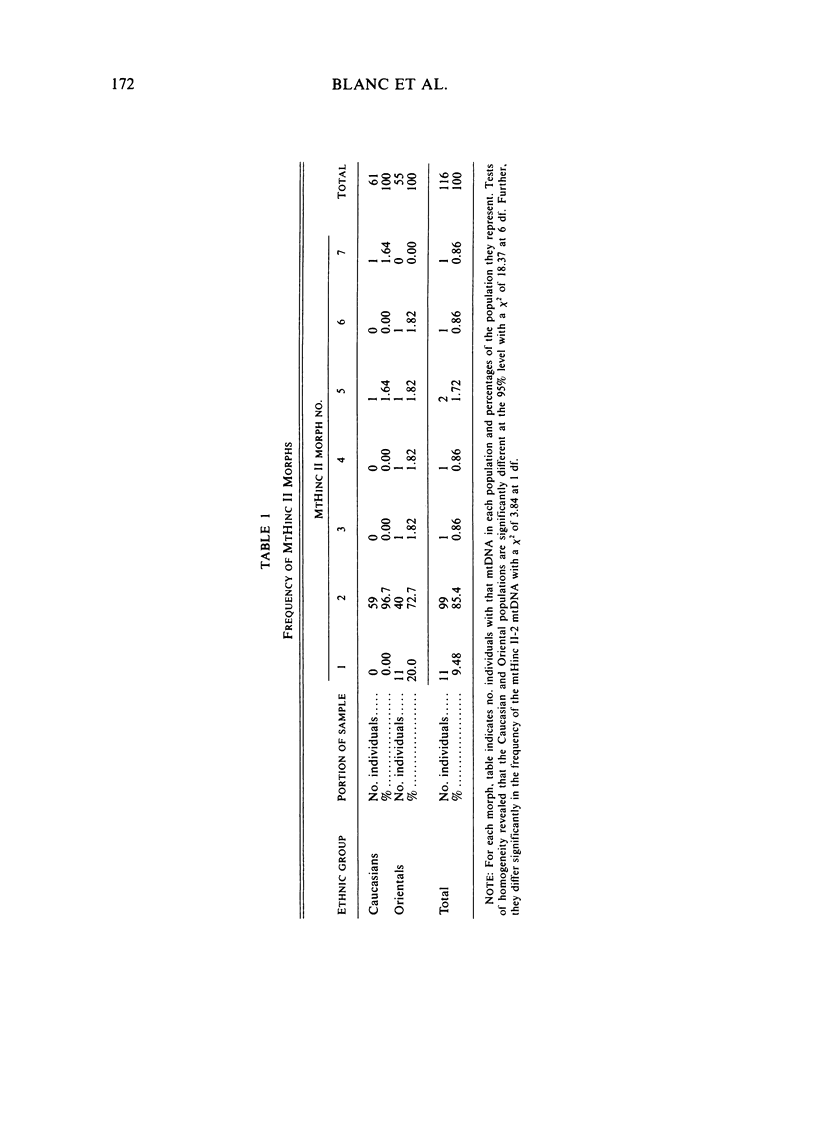
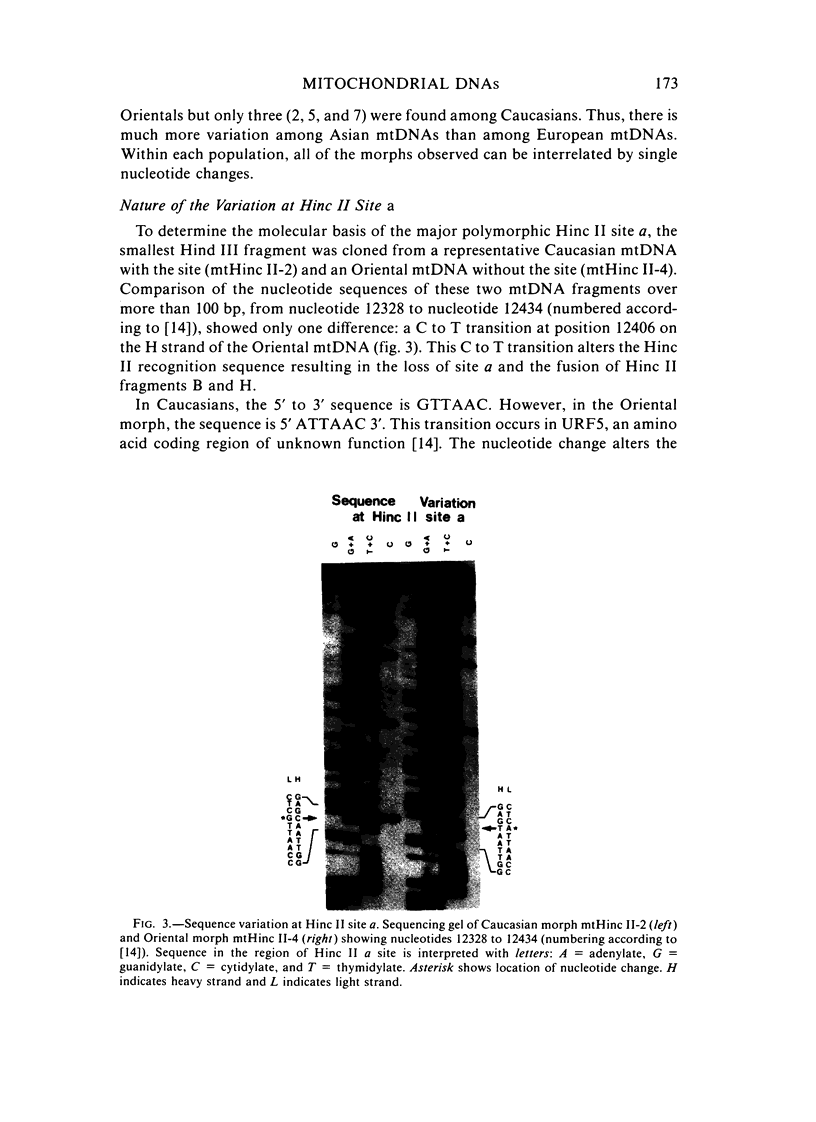
The mitochondrial DNAs (mtDNAs) from 116 Oriental and Caucasian blood samples were analyzed for their Hinc II restriction endonuclease cleavage patterns using Southern analysis and 32P human mtDNA probes. Seven distinct patterns were found, all of which could be interrelated by single nucleotide changes. The predominant pattern (mtHinc II-2) was found in 97% of the Caucasians and 73% of the Orientals. This mtDNA morph had one more Hinc II site than did the second most common morph (mtHinc II-1), which was found only in 20% of the Orientals. Three additional patterns were in a single Oriental sample, a fourth in a single Caucasian sample, and a fifth in one member of each population. The polymorphic site that differentiated mtHinc II-1 and mtHinc II-2 was cloned and sequenced. A single nucleotide change was found that created an Hinc II site and changed the amino acid sequence of the URF5 gene. Comparison of these sequences with those of other primates [15] revealed that the Asian mtHinc II-1 and mtHinc II-4 mtDNAs were identical in this region with those of chimpanzees and orangutans. These results suggest that the Asian mtHinc II-1 mtDNA may have been ancestral to other human mtDNAs.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Blanc H., Wright C. T., Bibb M. J., Wallace D. C., Clayton D. A. Mitochondrial DNA of chloramphenicol-resistant mouse cells contains a single nucleotide change in the region encoding the 3' end of the large ribosomal RNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3789–3793. doi: 10.1073/pnas.78.6.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M., George M., Jr, Wilson A. C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M. Polymorphism in mitochondrial DNA of humans as revealed by restriction endonuclease analysis. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3605–3609. doi: 10.1073/pnas.77.6.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M., Prager E. M., Wang A., Wilson A. C. Mitochondrial DNA sequences of primates: tempo and mode of evolution. J Mol Evol. 1982;18(4):225–239. doi: 10.1007/BF01734101. [DOI] [PubMed] [Google Scholar]
- Castora F. J., Arnheim N., Simpson M. V. Mitochondrial DNA polymorphism: evidence that variants detected by restriction enzymes differ in nucleotide sequence rather than in methylation. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6415–6419. doi: 10.1073/pnas.77.11.6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denaro M., Blanc H., Johnson M. J., Chen K. H., Wilmsen E., Cavalli-Sforza L. L., Wallace D. C. Ethnic variation in Hpa 1 endonuclease cleavage patterns of human mitochondrial DNA. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5768–5772. doi: 10.1073/pnas.78.9.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris S. D., Brown W. M., Davidson W. S., Wilson A. C. Extensive polymorphism in the mitochondrial DNA of apes. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6319–6323. doi: 10.1073/pnas.78.10.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris S. D., Wilson A. C., Brown W. M. Evolutionary tree for apes and humans based on cleavage maps of mitochondrial DNA. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2432–2436. doi: 10.1073/pnas.78.4.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles R. E., Blanc H., Cann H. M., Wallace D. C. Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6715–6719. doi: 10.1073/pnas.77.11.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles R. E., Stroynowski I., Wallace D. C. Characterization of mitochondrial DNA in chloramphenicol-resistant interspecific hybrids and a cybrid. Somatic Cell Genet. 1980 Jul;6(4):543–554. doi: 10.1007/BF01539155. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Piazza A., Menozzi P., Cavalli-Sforza L. L. Synthetic gene frequency maps of man and selective effects of climate. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2638–2642. doi: 10.1073/pnas.78.4.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter S. S., Newbold J. E., Hutchison C. A., 3rd, Edgell M. H. Specific cleavage analysis of mammalian mitochondrial DNA. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4496–4500. doi: 10.1073/pnas.72.11.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]